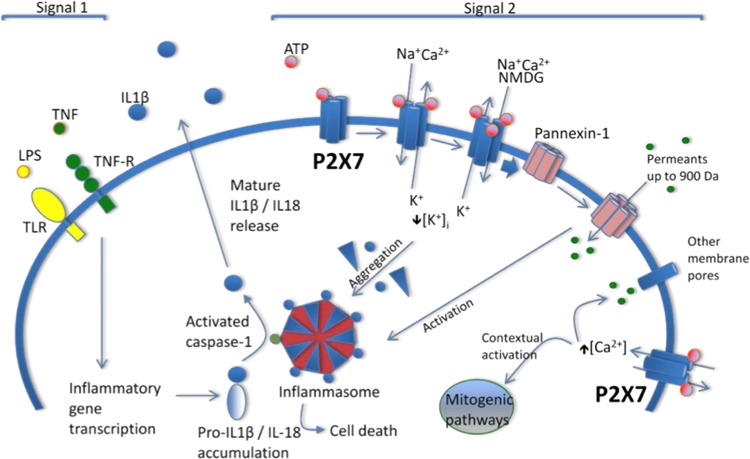
FIGURE 10.
A model of P2X7 receptor participation in cell proliferation, inflammation, and cell death in an archetypal cell, typically, though not exclusively, an immune cell, e.g., a macrophage. Release of mature interleukin (IL)-1β from macrophages requires a cellular priming signal delivered via Toll-like receptors in the case of microbial infection or inflammatory cytokine receptors (e.g., tumor necrosis factor receptor) in sterile inflammation, which leads to inflammatory gene transcription and accumulation of pro-IL-1β (‟Signal 1ˮ). P2X7 receptor activation requires relatively high local concentrations of ATP (in the high micromolar to millimolar range) usually released following cell injury, although localized cell surface concentrations may be sufficiently high in other settings. P2X7 receptor stimulation leads to an initial inorganic cation permeability when two ATP binding sites are occupied, progressing to pore formation when all three binding sites are occupied. An associated decrease in intracellular potassium is required for assembly of the NLRP3 inflammasome, the central caspase-1 activating platform. The P2X7 receptor then triggers opening of large-diameter membrane pores, including the pannexin-1 hemichannel. This event leads through still undefined mechanisms to activation of caspase-1, rapid maturation and release of IL-1β, and the related cytokine IL-18, and cell death (“Signal 2”). Elevations of cytosolic calcium in the absence of large membrane pore opening are thought to underlie the seemingly paradoxical mitogenic effect to P2X7 receptor activation seen in some settings (e.g., tumor growth). This P2X7 receptor-activated processes are believed to be important in a number of inflammatory diseases, including nephropathies in which inflammation, proliferation, and cell death may play a part, for example, glomerulonephritis, diabetic nephropathy, and polycystic kidney disease. See text for details. LPS, lipopolysaccharide; TNF, tumor necrosis factor; TLR, Toll-like receptor; TNF-R, tumor necrosis factor receptor; NMDG, N-methyl-d-glucamine. [Modified from Booth et al. (42), with permission from Dustri-Verlag.]