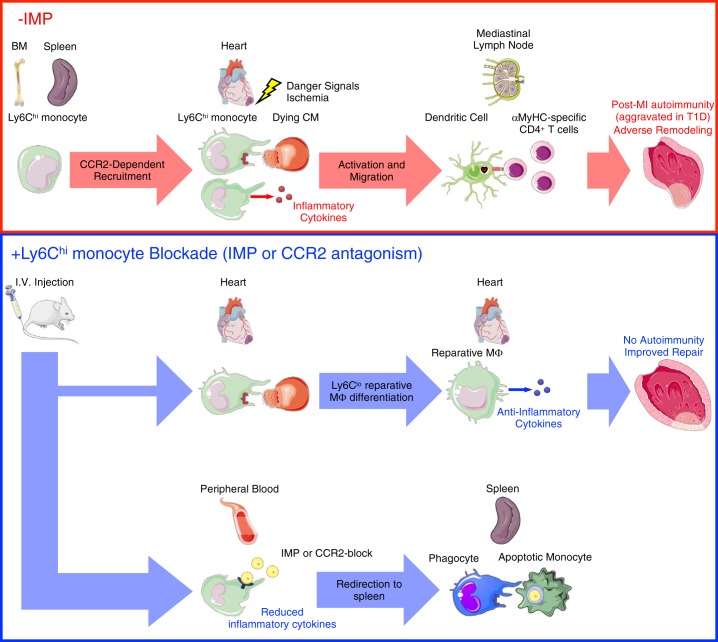
Fig. 4.
Monocytes prime autoreactive T cells after myocardial infarction (MI). MI causes release of danger signals and self-antigens from dying cardiomyocytes (CM). Ly6Chi monocytes are then released from the bone marrow (BM) and spleen into the peripheral blood and are recruited to the heart. Within the infarct, Ly6Chi monocytes produce inflammatory cytokines and phagocytose-damaged tissue and dying CM. In autoimmune-susceptible individuals, including patients with type 1 diabetes (T1D), persistent inflammatory LyC6hi monocytosis promotes infiltration and activation of antigen-presenting cells with phagocytosed cardiac self-antigen. Migration of mature antigen-presenting cells (dendritic cells) with engulfed cardiac self-antigen to the mediastinal lymph node and spleen leads to priming of cardiac myosin-specific CD4+ T cells and a pathological autoimmune response directed against the heart. This culminates in adverse tissue remodeling and progression to heart failure. Targeting monocyte recruitment to the heart using immune modifying nanoparticles (IMP) redirects the majority of Ly6Chi monocytes to the spleen for their apoptotic removal, while permitting a minority of these cells to infiltrate the infarct, mediate clearance of tissue debris, and differentiate into Ly6Clo reparative macrophages. This limits inflammation and both the release of cardiac self-antigen and its presentation by antigen-presenting cells in lymphoid organs, leading to improved cardiac repair after MI. α-MHC, α-myosin heavy chain; CCR2, C-C motif chemokine receptor type 2; Mϕ, macrophage.