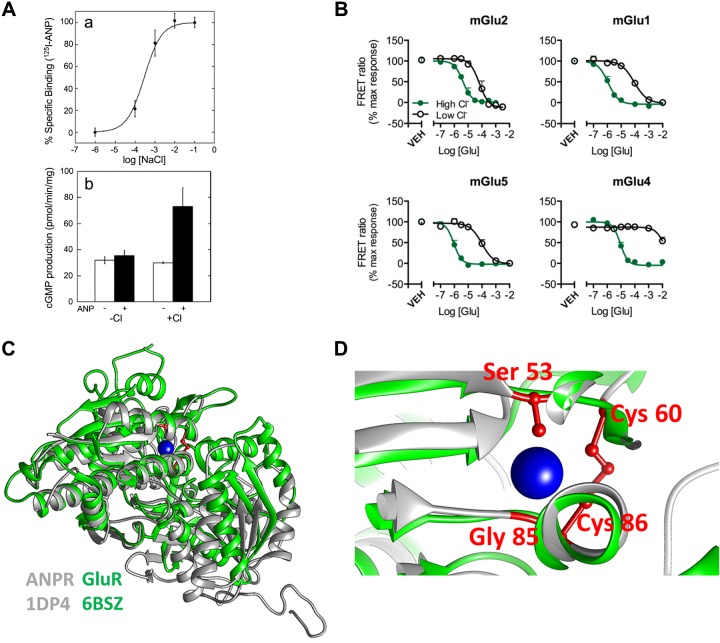
Fig. 1.
Signaling by [Cl−]out: effect of [Cl−]out on ligand-receptor interaction. A: effect of [Cl−]out on atrial natriuretic peptide (ANP) binding was measured in Chinese hamster ovary (CHO) cells transfected with the full-length ANP receptors. Isolated membranes were incubated in media containing the indicated Cl− concentrations and 125I−-ANP to measure ANP binding to the full-length ANP receptor (a). Isolated membranes from CHO cells transfected with the full-length ANP receptors were used to measure stimulation of guanylyl cyclase activity by ANP in the presence and absence of 100 mM NaCl (b). Results are taken from Ref. 65. B: group II and III mGlu receptors (mGluRs) were cotransfected with a chimeric Gi/Gq protein. The receptors were labeled with blue and green fluorescent tags for measurement of FRET. Labeled cells incubated in solutions containing [Cl−]out of 154.6 mM (green) to 2 mM (black) and FRET between Group II and III mGluRs in response to glutamate stimulation was measured. Shown are the changes in the indicated mGluRs conformation in response to receptor stimulation. Results are taken from Ref. 102. C and D: crystal structures of the rat ANP receptor (ANPR: gray; PDB_ID: 1DP4) and human mGlu8 receptor (GluR: green; PDB_ID: 6BSZ) (C) were obtained from the protein data bank website. The models were aligned and visualized using UCSF Chimera 1.11 (77). In D, a closeup of the ANPR chloride binding site residues are shown in red and the chloride ion in blue, with the mGlu8 Cl− binding site in green.