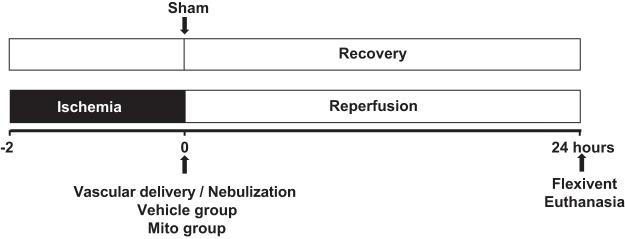
Fig. 1.
Experimental protocol. C57BL/6J male mice (8–10 wk, n = 36) were used for all experimental groups. Mice underwent left thoracotomy and the left pulmonary hilum was occluded at the end of expiration for 2 h using a microvascular clamp to create ischemia. At the end of the ischemia period, the clip was removed to allow for reperfusion, and the animals were randomized to receive either Vehicle or Vehicle containing mitochondria delivered either by vascular delivery (Mito V) via the pulmonary artery or by aerosol delivery (Mito Neb) via the trachea via nebulization. In the vascular delivery group at the beginning of reperfusion, either 1 × 108 mitochondria in 0.3 mL respiration buffer (Mito V, n = 6) or 0.3 mL respiration buffer (Vehicle V, n = 7) was injected as a bolus antegrade via the left pulmonary artery using a tuberculin syringe with a 40 G needle. In the nebulization group at the beginning of reperfusion, either 3 × 108 mitochondria in 90 μL respiration buffer (Mito Neb, n = 6) or 90 μL respiration buffer (Vehicle Neb, n = 6) were delivered to the whole lung over 40 s using the Aeroneb ultrasonic nebulizer following delivery protocol: 10 s nebulization followed by 1 min of regular mechanical ventilation, repeated four times. The mice were allowed to recover and returned to their cage for 24 h. Sham control mice (Sham, n = 5) underwent thoracotomy without hilar clamping and were mechanically ventilated for 2 h before returning to the cage. Following 24 h recovery, lung mechanics were assessed using FlexiVent system, bronchoalveolar lavage was obtained and lungs were collected for analysis.