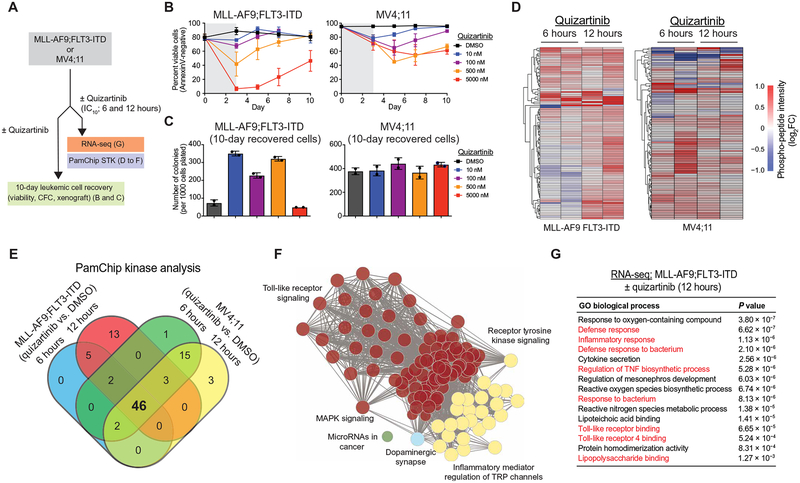
Fig. 1. FLT3-ITD AML develops adaptive resistance and activates innate immune pathways after FLT3i treatment.
(A) Overview of experimental design to evaluate adaptive resistance. CFC, colony-forming cell assay. (B) MLL-AF9;FLT3-ITD or MV4;11 cells were cultured with quizartinib for 3 days and then replated in fresh medium, and cell viability was measured by AnnexinV staining. Values are expressed as means ± SEM from three biological replicates. (C) After 10 days in liquid culture [from (B)], the remaining viable cells were plated in methylcellulose, and colony formation was determined after 7 days. Values are expressed as means ± SD from two biological replicates. (D) Serine-threonine kinase (STK) PamChip analysis was performed on protein lysates isolated from MLL-AF9;FLT3-ITD and MV4;11 cells treated with quizartinib (0.3 nM) for 6 and 12 hours. Hierarchical clustering analysis was performed on differentially phosphorylated peptides in the indicated groups relative to DMSO (two biological replicates). (E) In-cell active kinases inferred from the phosphorylated peptides (STK PamChip) are shown for each of the indicated conditions. (F) Pathway enrichment of differential in-cell kinase activity in MLL-AF9;FLT3-ITD and MV4;11 cells treated with quizartinib for 6 and 12 hours was determined using Panther. (G) Pathway enrichment of differentially expressed genes (>2-fold, P < 0.05) in MLL-AF9;FLT3-ITD cells treated with quizartinib for 12 hours was determined using ToppGene (n = 3 per group).