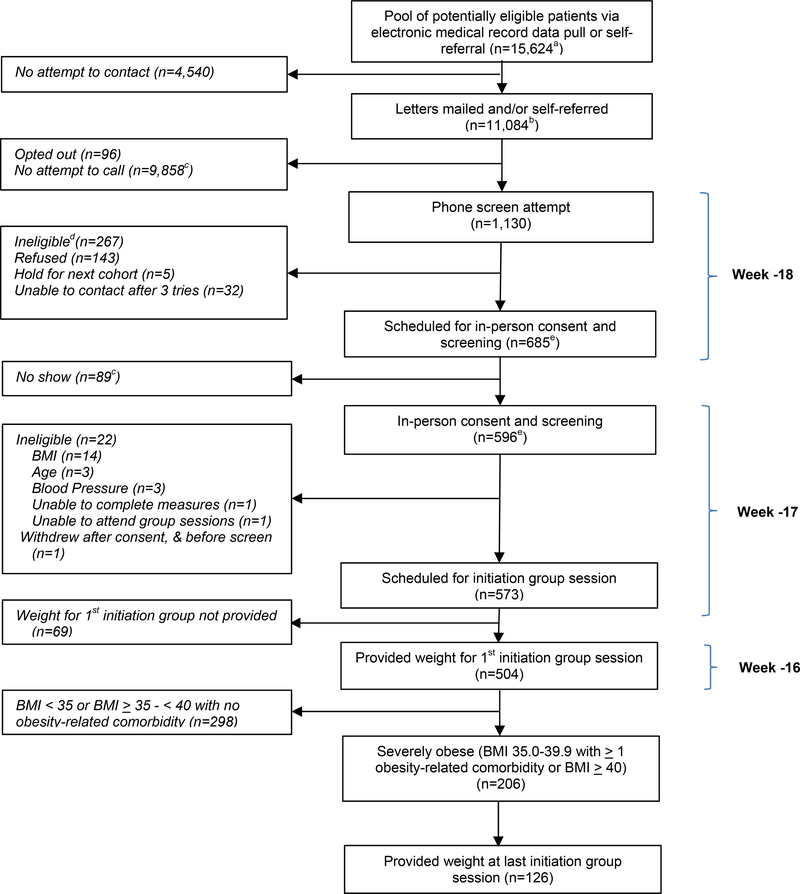
Figure 1. Patient Flow for Participants with Severe Obesity.
a Exclusion criteria determined by data pull included most recent serum creatinine >2.0 mg/dL in men or >1.7 mg/dL in women; liver disease; type 1 diabetes; most recent hemoglobin A1c, in past six months ≥12%; average systolic blood pressure over the past year of ≥ 160 mmHg and most recent BP ≥ 160 mmHg; history of weight loss surgery, dementia, severe psychiatric illness, or substance abuse
b N=10,807 were mailed letters; n=38 were mailed letters as well as being self-referred; n=239 were self-referred with no letter sent
c Obtained by subtraction
d Potential reasons for ineligibility assessed by telephone included: body mass index (BMI) <30 kg/m2 based on self-reported weight and height (reduced threshold to allow for error in reporting); self-reported age < 18 or > 75; weight loss ≥10 pounds in the previous 3 months; current enrollment in a lifestyle program; history of weight loss surgery; current use of weight loss medication or appetite suppressant; pregnancy or plan to become pregnant in upcoming 6 months, breastfeeding, or lack of birth control if premenopausal (female); organ transplant recipient; type 1 diabetes; heart disease with new treatment in past 3 months; liver disease; cancer not in remission; pacemaker or defibrillator due to use of bioelectronic impedance scale; major depression or emotional problems that would prevent following a diet closely or interacting with others in a group environment; illicit drug use or alcohol problems in the past year; inability to stand for study measurements; desire to lose weight; agreement to attend study visits; access to telephone; reliable transportation
e N=2 of the n=267 ineligibles at phone screen (1 due to BMI, and 1 due to age) are included in both the “Scheduled for in-person consent and screening” and “In-person consent and screening” boxes. One was ineligible at phone screen due to BMI < 30 kg/m2, but then was erroneously re-screened in-person and excluded at that point for the same reason. The second was listed as excluded due to age > 75 at both phone and in-person screen. Both exclusions were erroneous as the patient was 75 at both time points; however, the patient was not included in study after the in-person screen.