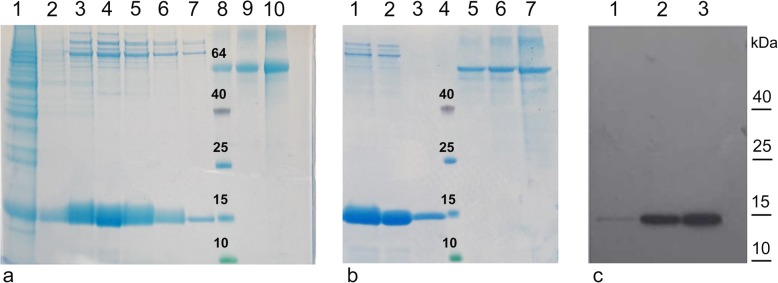
Fig. 4.
Purification and characterization of truncated recombinant rBacCPA250–363H6. a Coomassie stained 12% SDS-PAGE analysis after Ni-NTA purification. Lane 1: fraction observed on washing the column, after the passage of the recombinant rBacCPA250–363H6; lane 2–7: Elution fractions; lane 8–10: 1 μg, 2 μg and 4 μg of BSA standard; lane 8: Protein molecular weight marker. b Coomassie stained 12% SDS-PAGE analysis after Ni-NTA purification and after the dialysis step in PBS buffer. Lane 1: Elution fractions before the dialysis step (10 μl); Lane 2: Elution fractions after the dialysis step (10 μl); Lane 3: Elution fractions after the dialysis step (1 μl); (c) Immunoblot analysis of the elution fractions performed after Ni-NTA purification using anti-His6-HRP conjugated MAb. Lane1–3: Elution fractions corresponding to lane 2, 3 and 4 of samples in Comassie (a). The protein molecular weight marker is shown on the right