Figure 1.
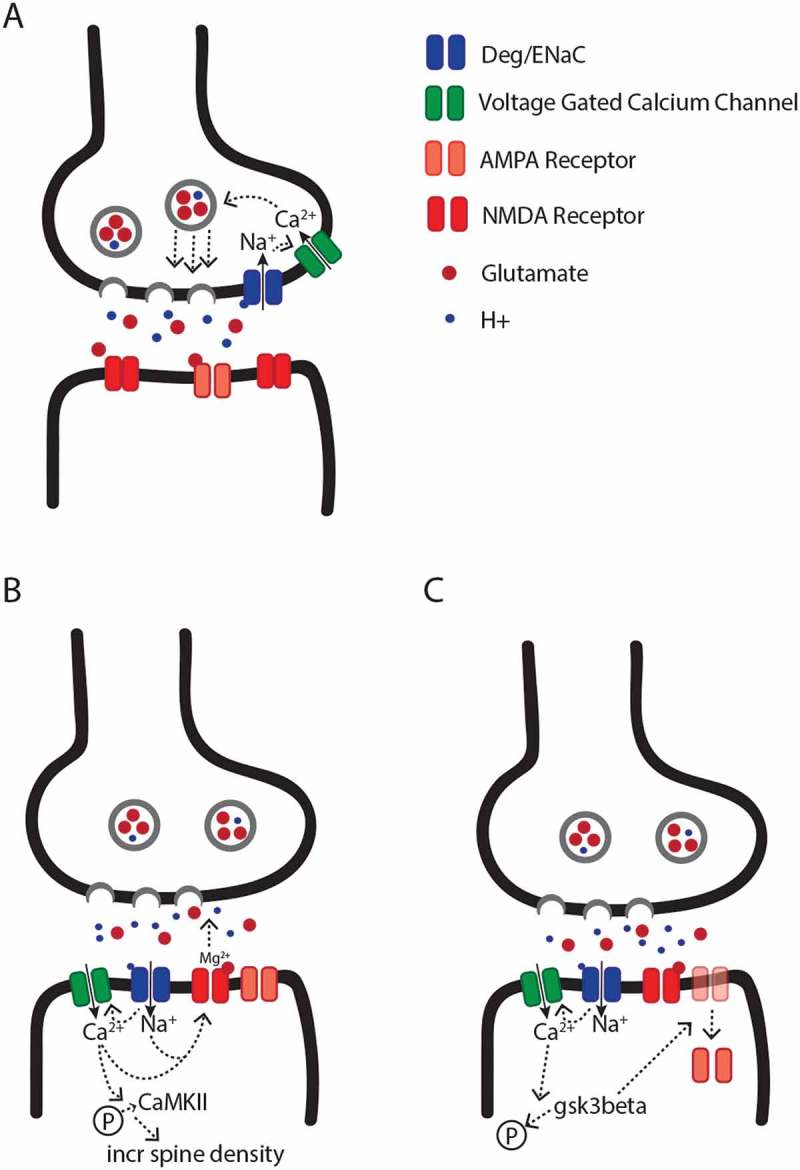
Models for the putative pre- and postsynaptic functions of DEG/ENaC channels. (a) Model for presynaptic DEG/ENaC function. The lumen of synaptic vesicles is acidic. Therefore, high frequency release of synaptic vesicles leads to an increase in proton concentration in the synaptic cleft. The lower pH leads to opening of presynaptic DEG/ENaCs, followed by a presynaptic sodium influx. Subsequently, local depolarization drives the opening of presynaptic voltage-gated calcium channels, and calcium-dependent synaptic vesicle release. (b) Model for postsynaptic DEG/ENaC mediated facilitation of synaptic activity. Upon the presynaptic release of vesicles, the synaptic cleft acidifies, which leads to an influx of cations directly through DEG/ENaC channels or indirectly via voltage-gated calcium channels, and the removal of the extracellular magnesium block from NMDA receptors. Subsequently, the DEG/ENaC-dependent calcium influx also induces the phosphorylation of CaMKII, which increases spine density. (c) Model for postsynaptic DEG/ENaC mediated depression of synaptic activity. As in (b), DEG/ENaC-mediated depolarization due to the acidification of the synaptic cleft leads to a calcium influx, which modulates the dephosphorylation and activation of GSK3β, which promotes internalization of postsynaptic AMPA receptors, and subsequently leads to long-term synaptic depression.
