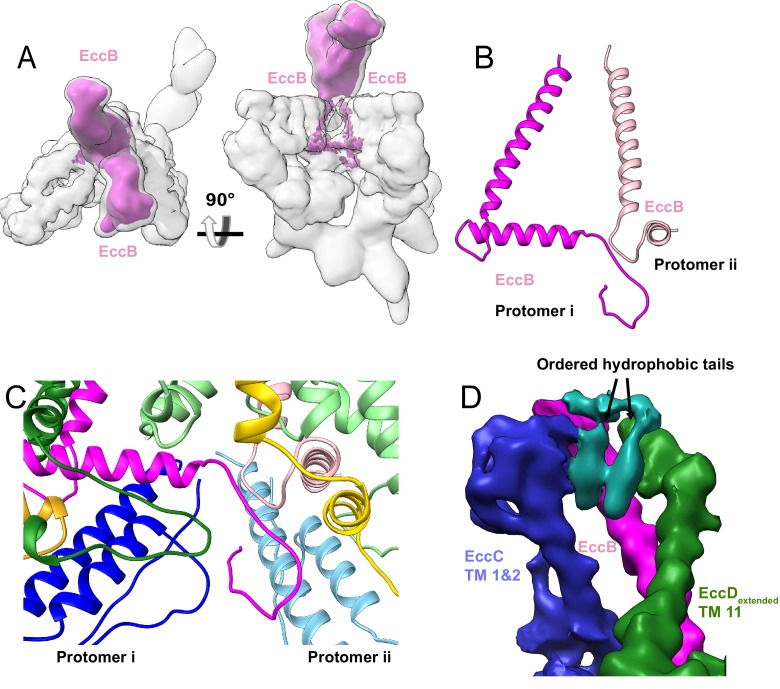
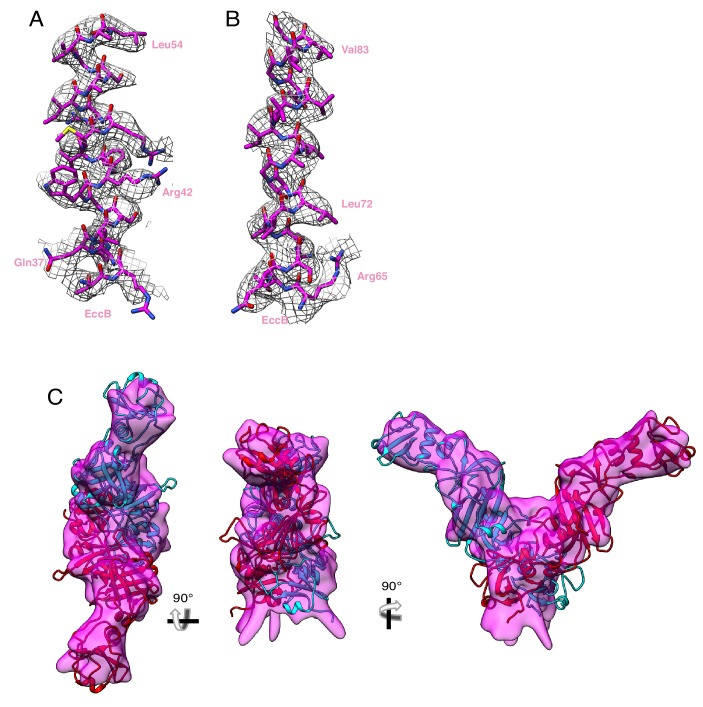
Figure 5. The periplasmic multimerization domain.
(A) EccB3 (pink) in the context of the overall ESX-3 dimer (gray transparency). EccB3 has a single-pass transmembrane domain which extends into a large periplasmic domain which was resolved at 5.8 Å resolution. (B) Atomic models of the EccB3 cytoplasmic and transmembrane domains, amino acids 14–93 and 32–93. (C) The N-terminus of EccB3 forms extensive cross-protomer contacts with EccC3 (blue), EccD3-bent (yellow), and EccD3-extended (green). (D) An unsharpened map of the ESX-3 dimer reveals ordered densities consistent with lipids or detergent molecules mediating the interactions between the EccB3 transmembrane helix (marked with a pink dot) and the EccC3 transmembrane helices.