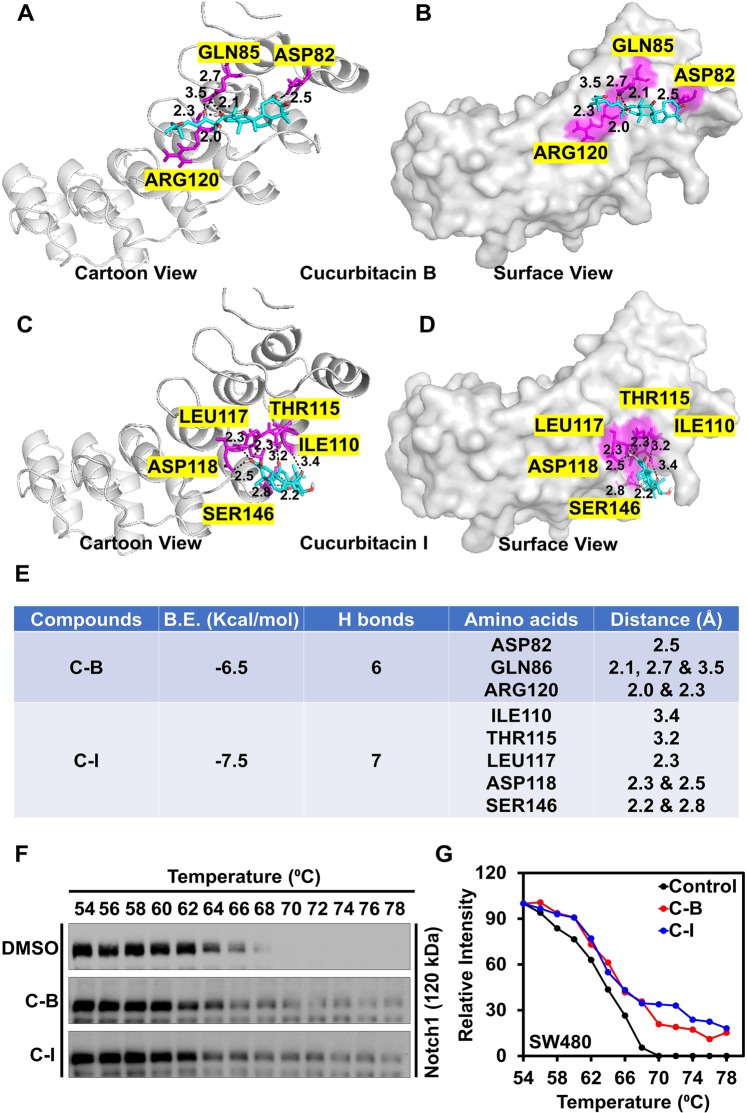
Figure 6.
Cucurbitacin B and I bind to Notch-1 protein. (A) Binding of C-B and C-I in the protein cavity of Notch ankyrin domains was assessed by molecular docking technique. C-B and C-I bind to the protein with the binding energy (B.E.) of −6.8 and −7.5 Kcal/mol respectively. Cartoon and surface models are shown in the figure for C-B (A and B) and C-I (C and D) respectively. (B) The docking results and consensus scores of C-B and C-I binding to Notch ankyrin domains are summarized. (C) Cellular thermal shift assay (CETSA) shows stabilization of Notch-1 protein after in the presence of C-B and C-I suggesting the potential binding. C-B and C-I were incubated with cell lysates from SW480 cell lines for two hours then subjected to thermal denaturation and evaluated using western blot. (D) Results from densitometric evaluation of CETSA assays. Notch-1 levels are represented relative to the band at 54 °C.