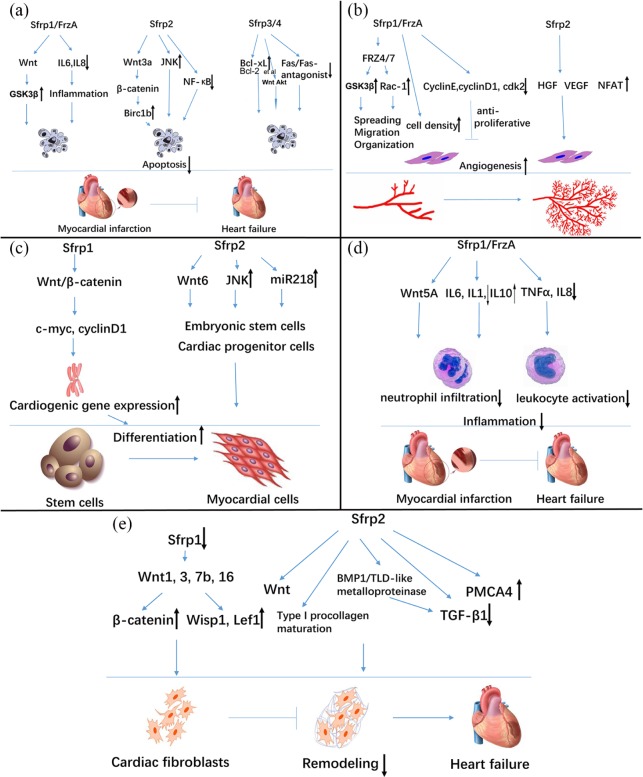
Figure 2.
Potential mechanisms of how Sfrps modulate cellular apoptosis, angiogenesis, differentiation, inflammatory process, and cardiac remodeling. (a) Sfrps inhibit apoptosis via a variety of pathways, including classic Wnt signaling (GSK3β, β-catenin), inflammation-induced apoptosis, such as NF-κB, IL6, and IL8, and interaction with the antiapoptotic protein Bcl-xL, Bcl-2, etc., and the Fas pathway. Sfrp4 might regulate both Wnt signaling and the Akt pathway. (b) Sfrps enhance spreading, migration, and organization of vascular endothelial cells, and increase release of growth factors (VEGF, hepatocyte growth factor) and cell density, thus promoting formation of a capillary network. However, in an antiproliferation role, Sfrps bind to cyclins to impair angiogenesis. (c) When Sfrps block c-myc and cyclin D1, expression of cardiac genes, and, subsequently, cardiogenesis and differentiation of cardiomyocytes, is promoted. Canonical and noncanonical Wnt pathways are indispensable in embryonic cardiogenesis and cardiac rehabilitation. (d, e) Sfrps negatively regulate activation of leukocytes and cardiac fibroblasts, and infiltration of neutrophils. This regulation is achieved by mediating Wnt signaling, tolloid-like metalloproteinase, TGF-β1, and calcium channels (PMCA4). This process reduces overproduction of ECM proteins and ameliorates ventricular remodeling and heart failure.
ECM, extracellular matrix; GSK-3β, glycogen synthase kinase-3β; NF-κB, nuclear factor κB; PMCA4, plasma membrane calcium ATPase 4; Sfrps, secreted frizzled-related proteins; TCF/LEF, T cell factor/lymphoid enhancer factor; TGF-β1, transforming growth factor β1; USF, upstream stimulatory factor; VEGF, vascular endothelial growth factor.