Research highlights
▸ We showed a longitudinal development of the prefrontal function. ▸ Development of cognitive shifting was related to inferior prefrontal activations. ▸ There might be individual differences in the development of prefrontal function.
Keywords: NIRS, Prefrontal cortex, Longitudinal study, Cognitive shifting, Children
Abstract
This is a longitudinal study on development of prefrontal function in young children. Prefrontal areas have been observed to develop dramatically during early childhood. To elucidate this development, we gave children cognitive shifting tasks related to prefrontal function at 3 years of age (Time 1) and 4 years of age (Time 2). We then monitored developmental changes in behavioral performance and examined prefrontal activation using near infrared spectroscopy. We found that children showed better behavioral performance and significantly stronger inferior prefrontal activation at Time 2 than they did at Time 1. Moreover, we demonstrated individual differences in prefrontal activation for the same behavioral tasks. Children who performed better in tasks at Time 1 showed significant activation of the right inferior prefrontal regions at Time 1 and significant activation of the bilateral inferior prefrontal regions at Time 2. Children who showed poorer performance at Time 1 exhibited no significant inferior prefrontal activation at Time 1 but significant left inferior prefrontal activation at Time 2. These results indicate the importance of the longitudinal method to address the link between cognitive and neural development.
1. Introduction
Over the last few decades, cognitive neuroscience research has revealed correlations between performance in cognitive tasks and brain activation in specific regions, such as the prefrontal regions. Various methodological approaches have been used, including neuropsychological, electrophysiological, and neuroimaging techniques (Dias et al., 1996, Konishi et al., 1998, Milner, 1963). Recently, there has been growing interest in the relationship between cognitive and brain development during the first few years after birth. Numerous studies have shown that cognitive development is indeed correlated with brain development during infancy and early childhood (Liu et al., 2009, Moriguchi and Hiraki, 2009, Rueda et al., 2005, Taga et al., 2003, Tsujimoto et al., 2004).
Most developmental cognitive neuroscience studies have relied on a cross-sectional design in which an investigator observes several age groups simultaneously. This approach has several advantages (e.g., it is easier to conduct the research), but it cannot be used to determine dynamic developmental changes (Kraemer et al., 2000, Magnusson and Casaer, 1993). Cross-sectional research can clarify the differences in brain activation and cognitive performance between two different age groups, but it cannot address how the differences occur and whether there are single or multiple paths of brain and cognitive development. Therefore, longitudinal approaches are needed to address these issues. Nevertheless, few longitudinal studies have been conducted in cognitive neuroscience research, especially in infants and young children, despite the understanding that the early phases of maturation during fetal development and childhood are the most dramatic and important (Toga et al., 2006).
The present study aimed to investigate longitudinal brain and cognitive development in young children, focusing on the prefrontal cortex. The prefrontal cortex is a brain region that subserves complex cognitive functions, such as cognitive shifting (Crone et al., 2006, Dias et al., 1996, Milner, 1963). Brain imaging studies in adults have shown that participants were likely to recruit inferior and dorsolateral prefrontal regions during cognitive shifting tasks, such as the Wisconsin Card Sorting Test (WCST) (Konishi et al., 1998, Monchi et al., 2001, Sumitani et al., 2006). However, recent studies have suggested that there are some individual differences in the laterality of activation during the WCST. In a near-infrared spectroscopy (NIRS) study, some participants recruited the left prefrontal areas during the WCST, whereas other participants showed right prefrontal activations (Sumitani et al., 2006). Moreover, neuropsychological research has revealed that patients with right lateral prefrontal damage as well as those with left lateral prefrontal damage showed impaired performance in the WCST task, but the latter showed less impairment (Stuss et al., 2000). Given this evidence, we believe that individual differences may exist in the laterality of prefrontal activations in young children and that these differences may be related to individual differences in behavioral performance on cognitive tasks.
Recently, extensive research has shown that the prefrontal cortex develops dramatically during early childhood. Behavioral studies have repeatedly shown that during preschool years, children's performance improves in neuropsychological batteries related to prefrontal function (Gerstadt et al., 1994, Luciana and Nelson, 1998, Zelazo et al., 1996). Moreover, there is some anatomical evidence that the prefrontal cortex develops during preschool years (Diamond, 2002, Giedd et al., 1999, Gogtay et al., 2004, Huttenlocher, 1990). Electrophysiological studies have shown that in children, the electroencephalogram pattern changes between the ages of 1 and 4 years in a working memory task (Bell and Wolfe, 2007) and between the ages of 4 and 6 years in a flanker task (Rueda et al., 2005). In addition, in a recent study, neuroimaging data have demonstrated functional development of the inferior prefrontal regions during preschool years in a cognitive shifting task (Moriguchi and Hiraki, 2009).
Although behavioral, anatomical, and electrophysiological research studies have included longitudinal evidence, no longitudinal neuroimaging data exist for early childhood (cf., school-aged children, Durston et al., 2006), and no research has examined whether there are single or multiple developmental paths for prefrontal function. Therefore, in the present study, we conducted a longitudinal study of the development of the prefrontal function. Specifically, the present study focused on the longitudinal development of prefrontal function between 3 and 4 years of age. We chose this age period because there is ample evidence that children of those ages develop higher order cognitive functions that may be closely related to prefrontal function, such as cognitive shifting and theory of mind (Garon et al., 2008, Liu et al., 2009, Wellman et al., 2001, Zelazo and Müller, 2002).
We used the dimensional change card sort (DCCS), which is widely used to index cognitive shifting in young children (Garon et al., 2008, Zelazo et al., 1996). The task was chosen because it has been repeatedly shown that children improve their performance on the DCCS task between 3 and 4 years of age (Kirkham et al., 2003, Moriguchi and Itakura, 2008, Zelazo et al., 1996). In the DCCS task, children are asked to sort cards that have two dimensions, such as color and shape. In the preswitch phase, children are asked to sort cards (e.g., red cups, blue stars) into trays with target cards (e.g., a blue cup, a red star) according to one rule (e.g., color). In the postswitch phase, children are asked to sort the cards according to a second rule (e.g., shape). Typically, 3-year-old children perseverate to the first rule, whereas 4- and 5-year-old children do not (Kirkham et al., 2003, Moriguchi et al., 2007, Zelazo et al., 1996).
Our previous cross-sectional neuroimaging study using NIRS with the DCCS task showed that 5-year-old children activated bilateral inferior prefrontal regions during the preswitch and the potswtich phases compared to a control phase, where children simply sorted blank cards into a tray (Moriguchi and Hiraki, 2009, but see also a functional magnetic resonance imaging [fMRI] study, Morton et al., 2009). Moreover, the NIRS study showed that 3-year-old children who committed perseverative errors (hereafter, the perseverate group) failed to activate the inferior prefrontal regions, whereas those who performed the tasks correctly (hereafter, the pass group) activated the right inferior prefrontal regions during the preswitch and postswitch phases. These results suggested that sustained right inferior prefrontal activations would be important for successful cognitive shifting during the DCCS tasks.
The present longitudinal study aimed to extend the findings from our cross-sectional study in two ways. First, we examined the longitudinal development of prefrontal activations during the preschool age. Although our previous cross-sectional study showed differences in behavioral performance and prefrontal activations during the DCCS tasks between 3- and 5-year-old children, it remained unclear whether the behavioral development was correlated with brain activation. Specifically, the present longitudinal study examined whether brain activation was changed when children's behavioral performance improved in the DCCS tasks. Second, individual differences in brain activation were also examined. Our previous study showed that children in the pass group exhibited right inferior prefrontal activations during the DCCS tasks, whereas children in the perseverate group failed to activate inferior prefrontal regions. Thus, we examined how inferior prefrontal activation changed in children from both groups while performing the same cognitive tasks.
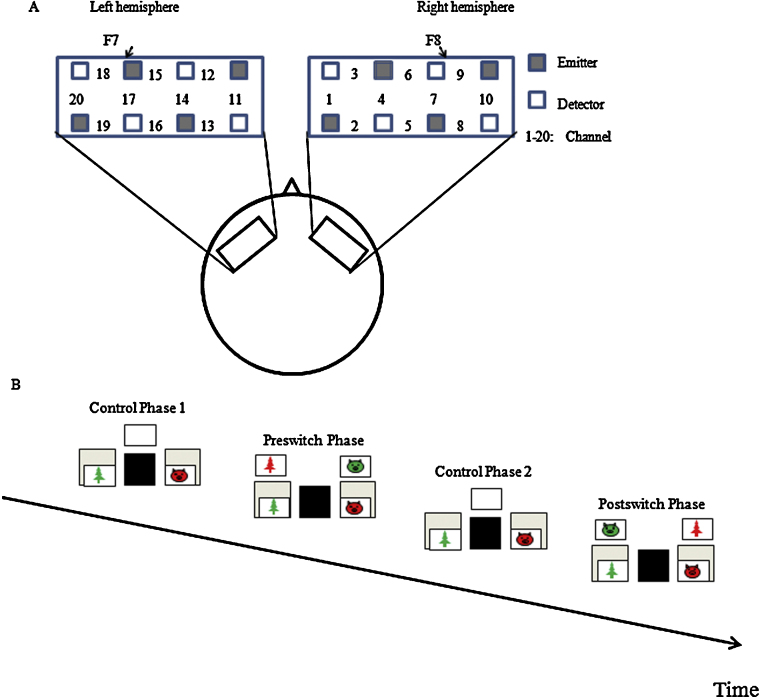
In the present study, we gave children the DCCS tasks and examined the developmental changes in prefrontal activations using an NIRS technique. Children were tested twice: at 3 years of age (Time 1) and at 4 years of age (Time 2). Given the previous NIRS results (Moriguchi and Hiraki, 2009), the region of interest was located at around F7/8 on the International 10/20 system (Fig. 1A), which corresponds to Brodmann areas 45/47 (Okamoto et al., 2004). Brain activation during the DCCS tasks was compared to activation during the control phases. In the control phases, children were provided blank cards and instructed to place the cards into an extra tray.
Fig. 1.
Experimental settings. (A) The NIRS probe was attached to the inferior prefrontal area. Each channel consisted of one emitter optode and one detector optode. The region of interest was located near F7/8, which corresponds to channels 15, 17, and 18 and channels 6, 7, and 9, respectively. (B) A sequence of the experiment.
2. Materials and methods
2.1. Participants
Fifteen right-handed 3-year-old children participated in this longitudinal study, but two girls were excluded from the analyses because they did not participate in the second testing. The mean age at first testing was 41.1 ± 4.0 months (mean ± standard deviation [SD]; age range, 37–47 months; six girls), and the average age at the second testing was 50.1 ± 4.1 months (age range, 46–55 months). The study was conducted in accordance with the principles of the Declaration of Helsinki. Parents provided written informed consent and were informed verbally of the purpose of the study and the safety of the NIRS experiment. The experiments were approved by the local ethics committee. The data at Time 1 were partly reported in Moriguchi and Hiraki (2009).
2.2. Behavioral tasks
Children participated in the experiment sitting on the floor. The behavioral tasks at Time 1 and Time 2 were almost the same except that different stimuli were used. Laminated cards (3.5 cm × 7.0 cm) that had two dimensions (shape and color) were used as stimuli. The task included target cards and test cards; target cards matched test cards in one dimension but did not match in the other dimension (e.g., a red pig, a green tree, red trees and green pigs. For details, see Appendix A). The experiments included eight pairs of target and test cards, each of which was different in shape and color. Four pairs of cards were used at Time 1, and the rest of the cards were used at Time 2. There were eight pairs of target trays, and each of the trays contained target cards. At each session, a different tray with a different set of cards was used. For the control task, blank cards and an extra tray were used. The extra tray was placed between two target trays (Fig. 1B). Basically, the task was children-paced, but the experimenter controlled the children's pace when providing the cards.
Children were given four consecutive test sessions at Time 1 and four sessions at Time 2. One session consisted of a control phase (control 1), a preswitch phase, a second control phase (control 2), and a postswitch phase. During the control phases, children were provided with blank cards and asked to place these cards into an extra tray (e.g., “All the cards here”). During the preswitch and postswitch phases, children were given instructions regarding the rules (e.g., “This is a shape game. All the pigs go here, and all the trees go there.”), and were asked to sort the cards. The rule order (color vs. shape first) was constant across the four sessions for each child, but the order was counterbalanced across children. Moreover, the rule order was constant across Time 1 and Time 2. In the experiment, the preswitch and postswitch phases were 25 s each; this included the time required to provide instructions concerning the rules (the first 5.5 s in each phase). Each control phase was 25 s.
The percentage of correct responses and the reaction times were analyzed. The reaction time was defined as the average time taken by the participants to sort one test card. The reaction time was analyzed with the video tapes. We recorded the reaction time for each trial of each participant.
2.3. NIRS recordings and analysis
NIRS is a technique for monitoring cerebral hemodynamics by measuring changes in the attenuation of near-infrared light passing through tissue (Firbank et al., 1998, Hoshi et al., 2001). The NIRS system used and the analyses were exactly the same for Time 1 and Time 2. A multichannel NIRS unit operating at wavelengths of 780, 805, and 830 nm (OMM-1080S; Shimadzu, Kyoto, Japan) was used to measure temporal changes in the concentrations of oxyhemoglobin (HbO2), deoxyhemoglobin (HbR), and total hemoglobin (HbT). One NIRS probe included eight optodes that comprised 10 channels (Fig. 1A). Each channel consisted of one emitter optode and one detector optode located 2 cm apart. The sampling rate at each channel was approximately 10 Hz.
The region of interest was located at around F7/8 on the International 10/20 system (Fig. 1A), which corresponds to Brodmann areas 45/47 (Okamoto et al., 2004). Given the low spatial resolution of NIRS, channels 6, 7, and 9 (right inferior prefrontal area) and channels 15, 17, and 18 (left inferior prefrontal area) were roughly regarded as F8 and F7, respectively (Moriguchi and Hiraki, 2009). The test sessions in which motion artifacts were revealed by the video recordings and NIRS data were discarded. Variations for each sample datum of HbO2 were calculated by subtracting a previous datum from a current datum. Channels in which a variation of more than 3 SDs was detected were excluded from further analysis. We rejected the trial where an artifact was detected. Ultimately, approximately 6% of the data from the children were excluded from the analyses. Of the three NIRS parameters measured, a change in the HbO2 concentration was considered to be the best indicator of brain activity (Hock et al., 1997, Toronov et al., 2001). For the validity, the other parameters were reported in our Supporting Information online (Figs. S1 and S2). Raw HbO2 data from individual channels without motion artifacts were low-pass filtered through a Savitzky-Golay filter (Savitzky and Golay, 1964). Following previous studies (Matsuda and Hiraki, 2006, Moriguchi and Hiraki, 2009), the raw data were converted into Z scores. The Z score was calculated using the mean value and the SD of the changes in HbO2 concentration during the control phases. Consequently, the mean value and SD during the control phases were respectively changed to Z scores of 0 and 1 in every channel.
Since children were given instructions regarding the rules during the first 5.5 s of the task phases, the last 19.5 s during the preswitch and postswitch phases (5.5–25 s) were analyzed. Average changes in HbO2 during the task phases were calculated for all channels and each subject. The significance of the differences between the changes in HbO2 for the baseline (the last 5 s of the control phases) and the task (preswitch or postswitch) was determined by a two-tailed Student's t test for each channel. We compared the preswitch phase to control 1 and compared the postswitch phase to control 2. As multiple comparisons were conducted, we applied a .001 alpha level of significance (two groups, two times, two tasks, and six channels). The differences between the pass group and the perseverate group as well as between Time 1 and Time 2 (i.e., comparisons of the activations during the preswitch phases at Time 1 to those at Time 2) for each channel were compared using a paired t test, and the significance level was set at p < .001.
Second analyses were conducted by comparing the last 5 s of the task phases to the last 5 s of the control phases at both Time 1 and Time 2. We conducted the analyses to control the differences of the durations between the control phases (5 s) and task phases (19.5 s) in the first analyses. The second analyses revealed the basically consistent patterns of the brain activations to those of the first analyses, although the effects were somewhat stronger in the second analyses than in the first analyses. Thus, in the next section, we reported the results of the first analyses.
3. Results
The behavioral results as well as the NIRS results at Time 2 were directly compared with those at Time 1 to assess the longitudinal developmental change in cognitive shifting and prefrontal activation in young children. Children were classified into the pass group or the perseverate group, according to whether they committed perseverative errors during at least one of the four assigned sessions at Time 1. In other words, even if a child committed perseverative errors in the first session, but not in other sessions, the child was classified into the perseverate group. The mean number of the sessions the children committed the perseverative errors was 2.0 (SD = 1.26, range 1–4).
3.1. Behavioral results
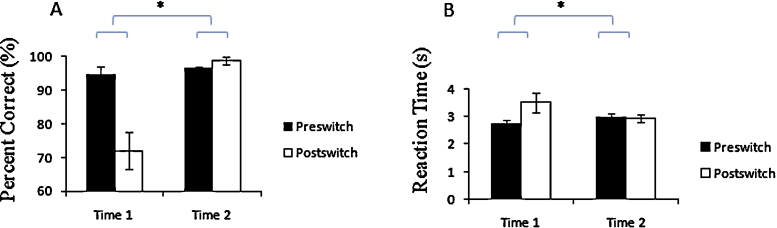
The mean percentage of correct responses by children at Time 1 was 94.9% in the preswitch phases and 72.1% during the postswitch phases. Six of the 13 children were classified into the perseverate group, and the remaining 7 were placed in the pass group. At Time 2, on average, children performed correctly 96.4% during the preswitch phases and 98.9% during the postswitch phases (Fig. 2A). None of the children committed perseverative errors at Time 2. The mean percentage of correct responses for the pass group and the perseverate group was 99.0% and 90.3% during the preswitch phases and 96.6% and 43.2% during the postswitch phases at Time 1, respectively. Also, the mean reaction time for the pass group and the perseverate group at Time 2 was 97.9% and 94.7% during the preswitch phases and 97.9% and 100.0% during the postswitch phases, respectively. The percentage of correct responses was analyzed using a 2 (Time 1 vs. Time 2) × 2 (preswitch vs. postswitch) ANOVA, and we observed a significant interaction of the percentage of correct responses [F (1, 95) = 11.258, p < .001, η2 = .11].
Fig. 2.
Behavioral results. (A) Percentage of correct responses and (B) reaction time during the preswitch and postswitch phases at Time 1 and Time 2. Error bars indicate standard error (SE). * p < .05.
Furthermore, we compared reaction times at Time 1 with reaction times at Time 2 and found that children required more time to sort the cards during the postswitch phase (M = 3.5 s) than during the preswitch phase (M = 2.7 s) at Time 1, whereas the reaction time at Time 2 was almost the same during both preswitch (M = 3.0 s) and postswitch phases (M = 3.0 s) (Fig. 2B). The reaction times for the pass group and the perseverate group were 2.3 s and 3.3 s during the preswitch phases and 2.8 s and 4.5 s during the postswitch phases at Time 1, respectively. Also, the mean reaction time for the pass group and the perseverate group at Time 2 was 2.7 s and3.3 s during the preswitch phases and 2.7 s and 3.2 s during the postswitch phases, respectively. We conducted a 2 (condition; pass vs. perseverate) × 2 (time; Time 1 vs. Time 2) × 2 (phase; preswitch vs. postswitch) ANOVA and found a main effect of condition [F (1, 86) = 17.128, p < .001, η2 = .13] and phase [F (1, 86) = 5.675, p < .02, η2 = .06] as well as a significant interaction between time and phase [F (1, 86) = 6.292, p < .02, η2 = .07]. Both accuracy and efficiency results therefore showed that children improved their behavioral performance of the cognitive shifting task between Time 1 and Time 2.
3.2. NIRS results
3.2.1. Whole-group analysis
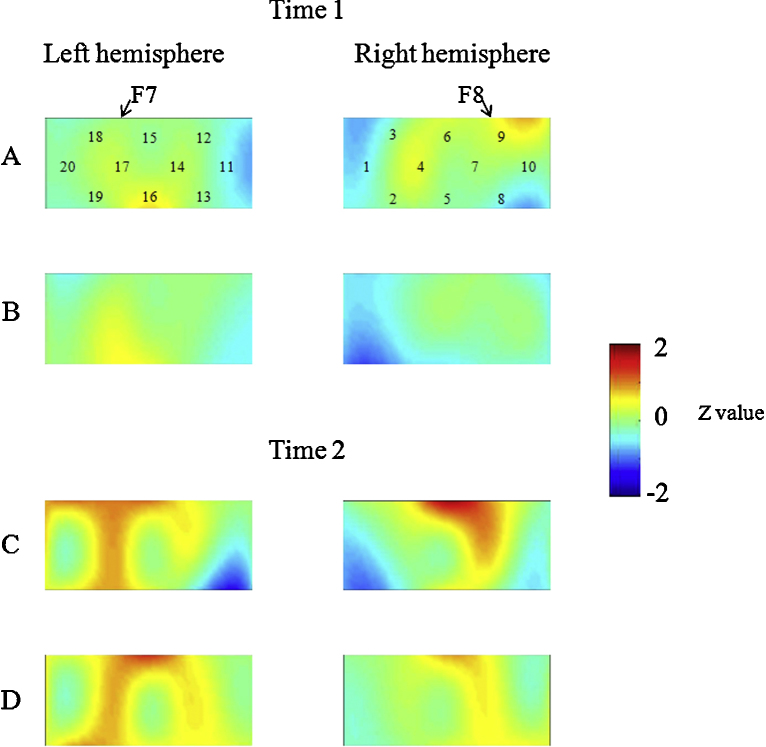
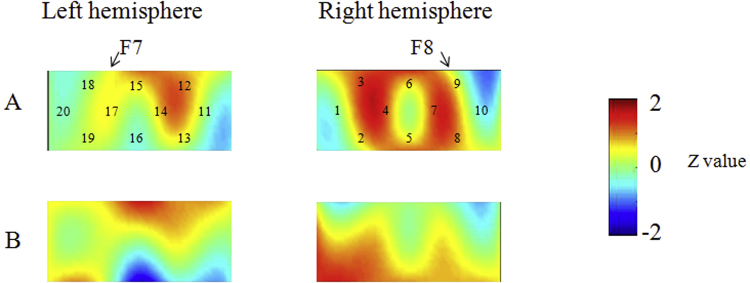
First, we examined whether there was a developmental change in brain activation for the whole group (Fig. 3). At Time 1, the results of the NIRS recordings revealed that children activated the right inferior prefrontal regions (channel 9) during the preswitch phase but failed to activate the bilateral inferior prefrontal areas during the postswitch phase as compared with control phases (Student's t test, p < .001). At Time 2, in the right (channels 6 and 7) and left (channels 15, 17, and 18) inferior prefrontal areas, the mean change in HbO2 was significantly higher during the preswitch phase than during the control phases. During the postswitch phase, children exhibited significant changes in HbO2 in the right (channel 9) and left (channels 15, 17, and 18) inferior prefrontal areas (p < .001). We then directly compared brain activation at Time 1 with that at Time 2 and found that children showed significant activation in the right and left inferior prefrontal areas during the preswitch (channels 6 and 7 and channels 15, 17, and 18) and postswitch phases (channels 6, 7, and 9 and channels 15, 17, and 18) at Time 2 as compared with Time 1 (paired t test, p < .001). Further analyses conducted to explore the difference between preswitch and postswitch phases are reported in our Supporting Information online.
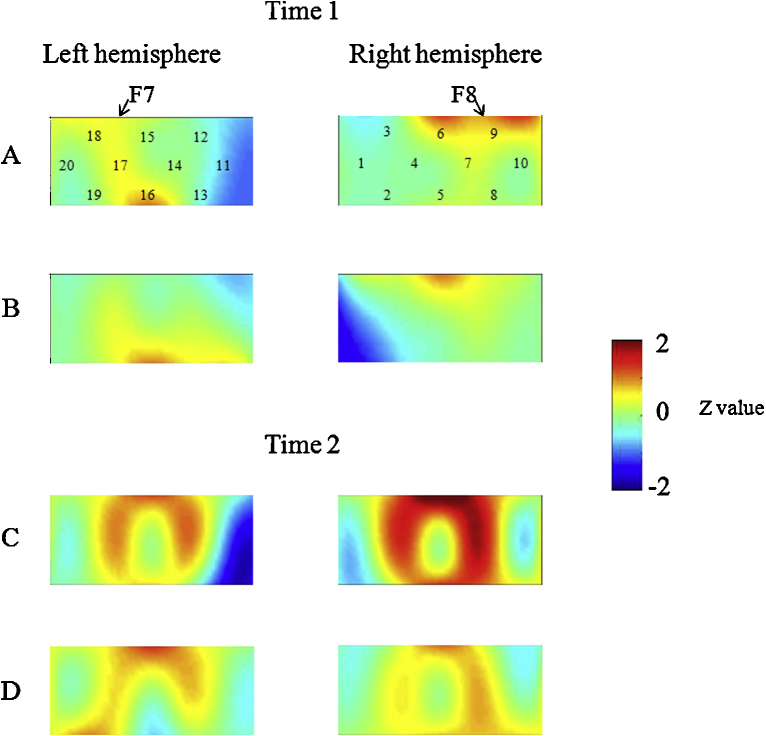
Fig. 3.
Data from children in the whole group at Time 1 (A, B) and Time 2 (C, D) during the DCCS task. Averaged overall data during the task phases compared with the control phases are shown. The numbers (1–20) indicate the channels of the NIRS probe. (A) Preswitch phase and (B) postswitch phase at Time 1. (C) Preswitch phase and (D) postswitch phase at Time 2.
3.2.2. Separate analyses
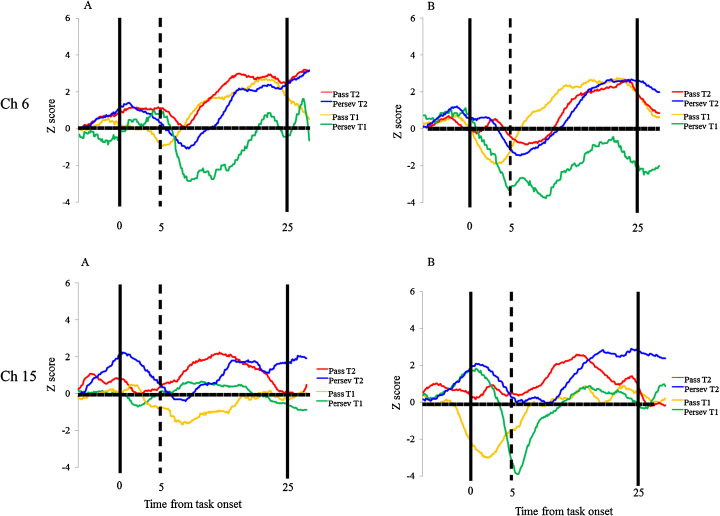
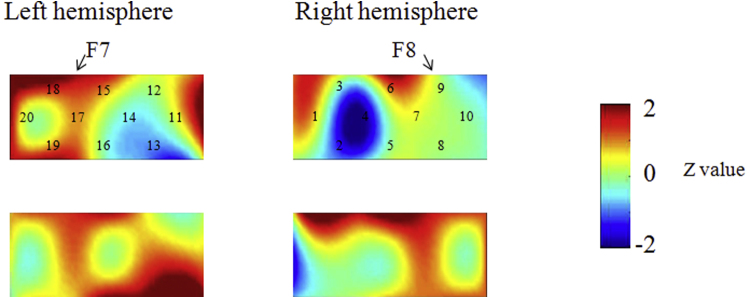
Next, we analyzed the children separately according to the perseverative errors observed at Time 1. First, we examined whether children in the pass group (n = 7) showed similar or different brain activations for Time 1 and Time 2. In terms of similarity in brain activation, children in the pass group showed significant right inferior prefrontal activation (channel 6) during the preswitch and postswitch phases at Time 1 and Time 2 as compared with the control phases (p < .001; Fig. 4, Fig. 5). In terms of differences in brain activation, children in the pass group activated the left inferior prefrontal areas during the preswitch (channel 15) and postswitch phases (channels 15, 17, and 18) at Time 2 but not at Time 1 as compared with the control phases (Fig. 4, Fig. 5). We then directly compared the brain activations at Time 1 with those at Time 2 and found that children in the pass group exhibited greater changes in HbO2 in the inferior prefrontal areas during the preswitch phase (channels 6, 7, and 15) and postswitch phase (channels 6 and 7 and channels 15 and 18) at Time 2 than they did at Time 1 (p < .001) (Fig. S3). These results revealed that during the DCCS tasks, children in the pass group exhibited right inferior prefrontal activations at Time 1, while they exhibited both right and left inferior prefrontal activations at Time 2.
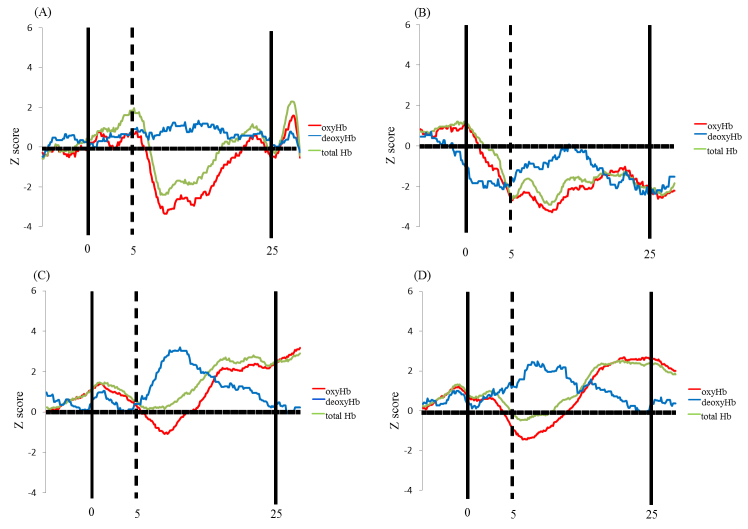
Fig. 4.
Temporal changes in the HbO2 concentration in the right (channel 6) and left (channel 15) inferior prefrontal areas during the experiment. (A) and (B) show the preswitch phases and the postswitch phases, respectively. Data of the group mean in children in the pass group at Time 1 (yellow line) and Time 2 (red line), and children in the perseverate group at Time 1 (green line) and Time 2 (blue line) are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
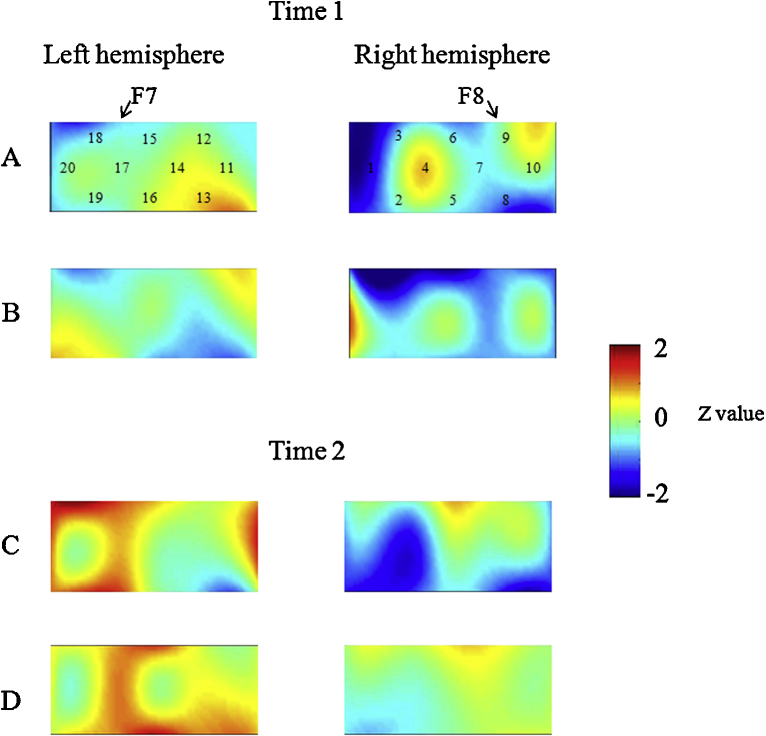
Fig. 5.
Data from children in the pass group at Time 1 (A, B) and Time 2 (C, D) during the DCCS task. Averaged overall data during the task phases compared with the control phases are shown. The numbers (1–20) indicate the channels of the NIRS probe. (A) Preswitch phase and (B) postswitch phase at Time 1. (C) Preswitch phase and (D) postswitch phase at Time 2.
Second, we examined whether children in the perseverate group showed different activations at Time 2 compared with Time 1. At Time 1, children in the perseverate group exhibited no significant HbO2 increases in either the right or left inferior prefrontal areas during the task phases compared with the control phases. Rather, they showed deactivations across the preswitch (channels 6, 15, and 18) and postswitch phases (channels 6, 9, and 15) (Fig. 4, Fig. 6). However, at Time 2, they showed significant increases in HbO2 in the left inferior prefrontal regions during the preswitch (channels 17 and 18) and postswitch phases (channels 15 and 18) (p < .001) compared with the control phases. They did not exhibit significant HbO2 increases in the right inferior prefrontal areas during either phase, although task phases seemed to affect the brain activations (Fig. 4, Fig. 6). Next, we compared the brain activations at Time 1 with those at Time 2 directly and found that children in the perseverate group exhibited significant HbO2 increases in the bilateral inferior prefrontal areas during the preswitch phase (channels 6 and 7 and channels 15, 17, and 18) and the postswitch phase (channels 6, 7, and 9 and channels 15 and 18) at Time 2 as compared with Time 1 (p < .001) (Fig. S4). These results revealed that children in the perseverate group exhibited no significant inferior prefrontal activations at Time 1, but showed significant left inferior prefrontal activations at Time 2 during the DCCS tasks.
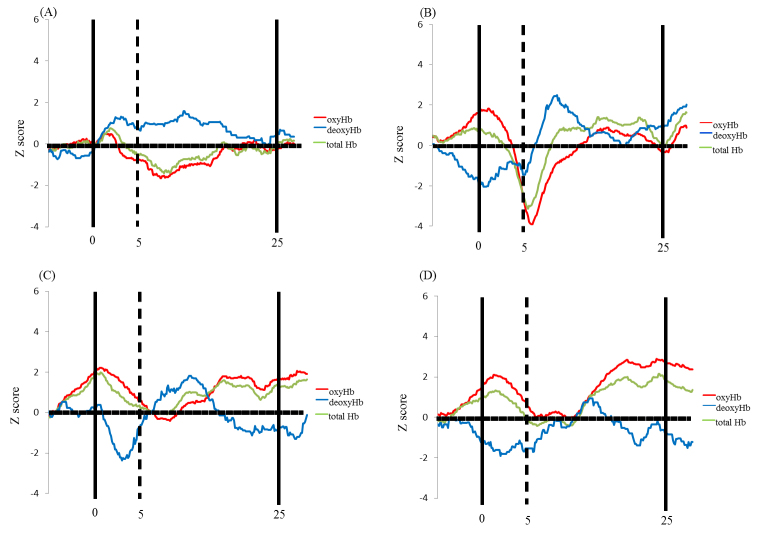
Fig. 6.
Data from children in the perseverate group at Time 1 (A, B) and Time 2 (C, D) during the DCCS task. Averaged overall data during the task phases compared with the control phases are shown. The numbers (1–20) indicate the channels of the NIRS probe. (A) Preswitch phase and (B) postswitch phase at Time 1. (C) Preswitch phase and (D) postswitch phase at Time 2.
Finally, we directly compared the brain activation in the pass group with those in the perseverate group. At Time 1, children in the pass group exhibited significantly stronger inferior prefrontal activations than did those in the perseverate group during the preswitch phase (channels 6, 7 and 9 and channels 15, 17, and 18) and the postswitch phase (channels 6, 7, and 9 and channels 15 and 17) (p < .001). Moreover, at Time 2, children in the pass group showed significantly greater activations compared with the perseverate group during the preswitch phase (channels 6, 7 and 9 and channels 15, 17, and 18) and postswitch phase (channel 17), while also showing significant deactivations during the postswitch phases (channel 9) (p < .001).
4. Discussion
Our longitudinal study clarified changes in behavioral performance and brain development during cognitive shifting occurring in young children. On the behavioral level, children showed a significant improvement in cognitive shifting as measured by the DCCS tasks. Six children committed perseverative errors at Time 1, but none of the children committed errors at Time 2. On the neural level, children showed a significant improvement in inferior prefrontal activations between Time 1 and Time 2. At Time 1, in general, children showed weaker inferior prefrontal activations across the DCCS tasks; at Time 2, children significantly activated bilateral inferior prefrontal regions during the preswitch phase and left inferior prefrontal regions during the postswitch phase.
These results support and extend our previous cross-sectional findings (Moriguchi and Hiraki, 2009). The previous study documented significant differences in behavioral performance and inferior prefrontal activations during the DCCS tasks between 3- and 5-year-old children. However, it was still unclear whether the behavioral differences were related to the inferior prefrontal activations. The present study suggests that the behavioral development observed during the DCCS tasks was correlated with the inferior prefrontal activations. The results implied that the development of cognitive shifting might be supported by the development of the inferior prefrontal cortex, although we could not discuss the causal relation between behavioral and neural changes from the results. More generally, this study is the first to provide longitudinal data indicating that children develop prefrontal activations between the ages of 3 and 4 years. Although some longitudinal anatomical and electrophysiological researches have demonstrated the development of prefrontal function during the preschool years (e.g., Rueda et al., 2005), little neuroimaging data are available regarding prefrontal development during this time period. Coupled with the behavioral, anatomical, and electrophysiological evidence, the neuroimaging data would contribute to our understanding of the development of the prefrontal cortex during early childhood.
In the present study, children generally showed stronger left inferior prefrontal activations at Time 2 than at Time 1. There are several possible interpretations regarding these laterality effects. One possibility is that children developed their hand-preference during the period of the study. That is, children at Time 2 may have tended to use the right hand to sort the cards compared with Time 1. However, we refute this possibility because even at Time 1, children showed a strong hand preference, and all of the children were right-handed and sorted the cards with their right hands. The second possibility is that children developed speech perception during the study period, especially processing of the sentences that communicated the experiment's instructions. It has been shown in adult fMRI studies that sentence processing may rely on the left frontal, temporal, and parietal areas (e.g., Friederici et al., 2003). However, the fact that even at Time 1, children performed the preswitch phase of the DCCS task perfectly may weaken this possibility. In the DCCS tasks, the instructions were fundamentally the same in both the preswitch and postswitch phases. This suggests that children at Time 1 understood the instructions for the experiment but failed to activate the left inferior prefrontal regions. The third possibility is that children may recruit inner speech to perform the tasks correctly. Kirkham et al. (2003) showed that labeling a new rule in the DCCS task may lead to better performance. Also, children may internalize their private speech during early childhood (Manfra and Winsler, 2006, Vygotsky, 1988). This evidence may suggest that children at Time 2 were more likely to engage inner speech to perform the tasks than they were at Time 1, which may have activated the left inferior prefrontal regions.
One advantage of the longitudinal design is that we could assess how behavioral development is related to brain development. When the children were classified into the pass group and perseverate group, analyses yielded interesting results. At Time 1, children in the pass group exhibited right inferior prefrontal activations across the preswitch and postswitch phases as compared with the control phases. At Time 2, they exhibited activations in both the right and left inferior prefrontal regions. The activation pattern was similar to that of 5-year-old children in a previous study who exhibited bilateral inferior prefrontal activations during the DCCS tasks (Moriguchi and Hiraki, 2009). It is likely that a developmental process exists whereby children first engage the right inferior prefrontal regions and then recruit the bilateral inferior prefrontal regions during the DCCS task.
Nevertheless, the story is not that simple. Children in the perseverate group showed a different developmental pattern. At Time 1, children in this group failed to engage the bilateral prefrontal regions during the preswitch and postswitch phases. Rather, their inferior prefrontal regions exhibited deactivations during the DCCS task. However, at Time 2 the children performed the DCCS tasks almost perfectly (as did children in the pass group at both Time 1 and Time 2), showing significant left, but not right, inferior prefrontal activations during the preswitch and postswitch phases as compared with the control phases. In terms of the right inferior prefrontal areas, children in the perseverate group showed changes between Time 1 and Time 2. At Time 1, these children showed deactivations in the right inferior prefrontal areas during the DCCS tasks, whereas these deactivations were not observed at Time 2. We believe that the differences in performance of the DCCS tasks between Time 1 and Time 2 may be due to the significant activations in the left inferior prefrontal areas or the “improvement” of the deactivations in the right inferior prefrontal regions.
In both interpretations, the results for children in the perseverate group at Time 2 were different from the results for children in the pass group at Time 1 and Time 2. The results for Time 1 and Time 2 suggested that unilateral (either right or left) inferior prefrontal activations may be important for successful performance in cognitive shifting tasks, such as the DCCS task. A previous study suggested that “sustained right inferior prefrontal activation might be crucial for successful cognitive shifting in young children” (Moriguchi and Hiraki, 2009, p. 6019). Instead, we suggest that sustained inferior prefrontal activations rather than sustained right inferior prefrontal activations may be crucial for successful cognitive shifting in young children. Taken together, these separate analyses suggest the existence of multiple paths in the development of the prefrontal activations observed during the cognitive shifting tasks. However, due to the small sample size in both analyses, these findings are, at present, inconclusive. Further research should be done to assess and confirm the present findings with a bigger sample size.
In general, our findings with regard to unilateral activations seem to be consistent with those of some previous studies in which participants recruited either right or left inferior prefrontal regions during cognitive shifting tasks. Adult brain imaging studies have suggested that there are individual differences in the laterality of the activation during the WCST; some participants recruited the left prefrontal areas, whereas other participants demonstrated right prefrontal activations (Sumitani et al., 2006). Bunge et al. (2002) reported that adult participants recruited right ventrolateral prefrontal regions during a flanker task, whereas school-aged children activated left ventrolateral prefrontal regions, suggesting that children may use different strategies compared with adults. Moreover, neuropsychological research has revealed that patients with right or left lateral prefrontal damage experienced difficulty with the WCST, but those with left lateral prefrontal damage (i.e., those with intact right prefrontal regions) showed less impairment (Stuss et al., 2000). This neuropsychological evidence may accord with the present results indicating that 3-year-old children in the pass group first recruited the right inferior prefrontal areas. In other words, right inferior prefrontal areas may be relatively dominant in cognitive shifting tasks, and left inferior prefrontal areas may support or compensate for right inferior prefrontal activations.
Finally, the fact that there might be multiple neural developmental paths in prefrontal function may contribute to our understanding of developmental disorders such as attention-deficit hyperactivity disorder (ADHD). It has been hypothesized that patients with ADHD may have functional deficits in the prefrontal cortex (Bush et al., 2005). However, it is still unclear when and how children with ADHD exhibit this deficit in prefrontal function. Children with ADHD may exhibit a delay in the maturation of prefrontal areas (Shaw et al., 2007). Nevertheless, given the results of the present study, it may be possible that prefrontal function follows a different developmental pathway in early childhood in children with ADHD compared with children undergoing typical development. Further research using longitudinal methods should be performed to address these issues.
Acknowledgements
This research was supported by grants from JSPS to Kazuo Hiraki and Yusuke Moriguchi. Also, this was supported by JST PRESTO program. We thank G. Matsuda, R. Matsunaka, and I. Shinohara for help with data collection. We also thank the parents and children who participated in this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2010.12.004.
Contributor Information
Yusuke Moriguchi, Email: moriguchi@juen.ac.jp.
Kazuo Hiraki, Email: khiraki@idea.c.u-tokyo.ac.jp.
Appendix A. Appendix
Stimuli used at Time 1 and Time 2
Time 1
Pair 1: a red star, a blue cup, red cups, and blue stars.
Pair 2: a green car, a yellow house, green houses, and yellow cars.
Pair 3: an orange face, a purple flower, orange flowers and purple faces.
Pair 4: a gray camera, a brown computer, gray computers, and brown cameras.
Time 2
Pair 1: a red pig, a green tree, red trees and green pigs.
Pair 2: a yellow bag, a blue truck, yellow trucks, blue bags.
Pair 3: a purple triangle, a brown circle, purple circles, and brown triangles.
Pair 4: a gray cloud, a white TV, gray TVs, and white clouds.
Appendix A. Supplementary data
References
- Bell M.A., Wolfe C.D. Changes in brain functioning from infancy to early childhood. Dev. Neuropsychol. 2007;31:21–38. doi: 10.1207/s15326942dn3101_2. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Vaela E.M., Seidman L.J. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol. Psychiatr. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Crone E., Donohue S., Honomichl R., Wendelken C., Bunge S. Brain regions mediating flexible rule use during development. J. Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry. In: Stuss D.T., Knight R.T., editors. Principles of Frontal Lobe Function. Oxford Univ. Press; New York: 2002. pp. 466–503. [Google Scholar]
- Dias R., Robbins T.W., Roberts A.C. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9:1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Firbank M., Okada E., Delpy D.T. A theoretical study of the signal contribution of regions of the adult head to near-infrared spectroscopy studies of visual evoked responses. Neuroimage. 1998;8:69–78. doi: 10.1006/nimg.1998.0348. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Ruschemeyer S.A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Garon N., Bryson S.E., Smith I.M. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gerstadt C.L., Hong Y.J., Diamond A. The relationship between cognition and action: performance of children 3.5–7 years old on a Stroop-like day-night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Lui H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., III, Harman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C., Villringer K., Muller-Spahn F., Wenzel R., Heekeren H., Schuh-Hofer S., Hofmann M., Minoshima S., Schwaiger M., Dirnagl U., Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored by means of near-infrared spectroscopy (NIRS): correlation with simultaneous rCBF-PET measurements. Brain Res. 1997;755:293–303. doi: 10.1016/s0006-8993(97)00122-4. [DOI] [PubMed] [Google Scholar]
- Hoshi K., Kobayashi N., Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J. Appl. Physiol. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Kirkham N.Z., Cruess L., Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Dev. Sci. 2003;6:449–476. [Google Scholar]
- Konishi S., Nakajima K., Uchida I., Kameyama M., Nakahara K., Sekihara K., Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat. Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Kraemer H.C., Yesavage J.A., Taylor J.L., Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Liu D., Sabbagh M.A., Gehring W.J., Wellman H.M. Neural correlates of children's theory of mind development. Child Dev. 2009;80:318–326. doi: 10.1111/j.1467-8624.2009.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Nelson C.A. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Magnusson D., Casaer P. Cambridge University Press; Cambridge: 1993. Longitudinal Research on Individual Development: Present Status and Future Perspectives. [Google Scholar]
- Manfra L., Winsler A. Preschool children's awareness of private speech. Int. J. Behav. Dev. 2006;30:537–549. [Google Scholar]
- Matsuda G., Hiraki K. Sustained decrease in oxygenated hemoglobin during video games in the dorsal prefrontal cortex: a NIRS study of children. Neuroimage. 2006;29:706–711. doi: 10.1016/j.neuroimage.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch. Neurol. 1963;9:90–100. [Google Scholar]
- Monchi O., Petrides M., Petre V., Worsley K., Dagher A. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J. Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Itakura S. Young children's difficulty with inhibitory control in a social context. Jpn. Psychol. Res. 2008;50:87–92. [Google Scholar]
- Moriguchi Y., Lee K., Itakura S. Social transmission of disinhibition in young children. Dev. Sci. 2007;10:481–491. doi: 10.1111/j.1467-7687.2007.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.B., Bosma R., Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage. 2009;46:249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Dan H., Sakamoto K., Takeo K., Shimizu K., Kohno S., Oda I., Isobe S., Suzuki T., Kohyama K., Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., Saccamanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitzky A., Golay M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964;36:1627–1639. [Google Scholar]
- Shaw P., Eckstrand K., Sharp W., Blumenthal J., Lerch J.P., Greenstein D., Clasen L., Evans A., Giedd J., Rapoport J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T., Levine B., Alexander M.P., Hong J., Palumbo C., Hamer L., Murphy K.J., Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Sumitani S., Tanaka T., Tayoshi S., Ota K., Kameoka N., Ueno S., Ohmori T. Activation of the prefrontal cortex during the Wisconsin Card Sorting Test as measured by multichannel near-infrared spectroscopy. Neuropsychobiology. 2006;53:70–76. doi: 10.1159/000091722. [DOI] [PubMed] [Google Scholar]
- Taga G., Asakawa K., Maki A., Konishi Y., Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toronov V., Webb A., Choi J.H., Wolf M., Michalos A., Gratton E., Hueber D. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med. Phys. 2001;28:521–527. doi: 10.1118/1.1354627. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S., Yamamoto T., Kawaguchi H., Koizumi H., Sawaguchi T. Prefrontal cortical activation associated with working memory in adults and preschool children: an event-related optical topography study. Cereb. Cortex. 2004;14:703–712. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- Vygotsky L.S. On inner speech. In: Franklin M.B., Barten S., editors. Child Language: A Reader. Oxford University Press; New York: 1988. pp. 181–187. [Google Scholar]
- Wellman H., Cross D., Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001;72:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D., Frye D., Rapus T. An age-related dissociation between knowing rules and using them. Cogn. Dev. 1996;11:37–63. [Google Scholar]
- Zelazo P.D., Müller U. Executive function in typical and atypical development. In: Goswami U., editor. Blackwell Handbook of Childhood Cognitive Development. Blackwell; Oxford, England: 2002. pp. 445–469. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.