Highlights
* Eye gaze guides infants’ attention and facilitates encoding of novel objects. * We compare the effects of the caregiver's gaze vs. a stranger's gaze using ERP. * 4-month-olds’ brain responses to objects are affected by the caregiver's gaze cues. * A stranger's gaze has no effect on infants’ responses to objects in this study. * The caregiver's eye gaze is more effective than a stranger's gaze for 4-month-olds.
Keywords: Infants, Event-related potentials (ERP), Eye gaze, Face processing, Face familiarity
Abstract
Previous research has shown that eye gaze affects infants’ processing of novel objects. In the current study we address the question whether presenting a highly familiar face vs. a stranger enhances the effects of gaze cues on object processing in 4-month-olds. Infants were presented pictures of the infant's caregiver and another infant's caregiver (stranger) either turning eye gaze toward an object next to the face or looking away from the object. Then objects were presented again without the face and event-related potentials (ERP) were recorded. An enhanced positive slow wave (PSW) was found for objects that were not cued by the caregiver's eye gaze, indicating that these objects required increased encoding compared to objects that were cued by the caregiver's gaze. When a stranger was presented, a PSW was observed in response to objects regardless of whether the objects were gaze-cued or not. Thus, the caregiver's eye gaze had a larger effect on infants’ object processing than the stranger's gaze. This suggests that at 4 months of age the caregiver's eye gaze is easier to process for infants, more salient, or both. The findings are discussed in terms of early social cognitive development and face processing models.
1. Introduction
Infants constantly encounter a large number of visual stimuli, familiar and novel objects and persons. Many questions remain concerning how preverbal infants structure their visual input, guide their attentional resources, and process novel stimuli. Recently it was shown that infants use cues of visual attention provided by adults when guiding their attention toward unfamiliar objects (Cleveland et al., 2007, Cleveland and Striano, 2007, Hoehl et al., 2008, Parise et al., 2008, Reid and Striano, 2005, Reid et al., 2004, Striano et al., 2006).
In a series of behavioral experiments Cleveland and colleagues investigated the effects of joint attention on infants’ encoding of novel objects in a naturalistic setting with a live experimenter (Cleveland et al., 2007, Cleveland and Striano, 2007). Infants were familiarized with one object either in a triadic interaction, in which the adult alternated gaze between infant and object including phases of mutual gaze, or in a control condition, in which the adult did not engage in eye contact with the infant. In a subsequent test phase the familiarized object was presented together with a novel object and novelty preference scores were compared across conditions. Infants at 7 and 9 months of age showed a significantly larger novelty preference for the unfamiliar object if they had been familiarized with the first object in a triadic interaction compared to the control condition.
In a study by Reid and colleagues (2004) 4-month-old infants saw a face shifting eye gaze either toward or away from a small object presented next to the face. Objects were then presented again without the face and infants’ brain responses (event-related potentials, ERP) to the objects were measured. Infants showed an increased positive slow wave (PSW) for objects that were not cued by the adult's eye gaze compared to objects that were cued by the adult's gaze. Amplitude of the PSW has been associated with updating the memory representation of a partially encoded stimulus (Nelson, 1994, Snyder, 2010). This suggests that in the study by Reid et al. (2004) objects that were not cued by the adult's eye gaze subsequently required increased processing compared to the cued objects, which were presumably more effectively encoded during the presentation with the face. This interpretation was later supported in a behavioral looking time study with 4-month-old infants (Reid and Striano, 2005). In this study a face shifted eye gaze toward one of the two objects that were displayed on the right and left side of the face on a computer monitor. Then the objects were presented again without the face and infants’ looking times for both objects were measured. Infants looked significantly more toward the non-cued compared to the cued object. This visual preference for the non-cued object was interpreted as a novelty preference due to the fact that non-cued objects were presumably less well encoded and consequently more novel to the infants compared to the cued objects. Twelve-month-olds also show a temporary visual preference for non-cued objects compared to cued objects in a similar paradigm (Theuring et al., 2007). These results suggest that others’ eye gaze helps infants to direct attention toward relevant objects, thereby facilitating memory encoding of the gaze-cued objects.
Based on these empirical findings the Directed Attention Model (DAM) of infant social-cognitive performance was developed (Hoehl et al., 2009, Reid and Striano, 2007). This information processing model describes the perceptual stages of processing social information which are required in order to respond appropriately to a social partner. The stages of this model involve the detection of a social agent (1), the identification of the social agent (2), the detection of the other's attention focus in relation to oneself (3), and the detection of the other's attention focus in relation to other objects or persons (4). According to this model the detection of another person's attention focus should be facilitated if the person is familiar to the observer because identification of a highly familiar face should be facilitated relative to a strange face and this should affect the subsequent processing stages. Though there is evidence that familiarity of a face enhances gaze cueing effects in female adults (Deaner et al., 2007), this assumption has not been tested empirically with infants.
Six-month-olds respond with an increased Negative central (Nc) component to presentations of their mother's face compared to a dissimilar looking stranger's face, indicating that infants recognize their mother's face and presumably direct increased attention toward their mother's face (de Haan and Nelson, 1997, de Haan and Nelson, 1999). There is behavioral evidence that infants discriminate their mother's face from other faces even few hours after birth (Bushnell et al., 1989). However, only a few studies have tested whether infants’ processing of social cues provided by a face is affected by familiarity. For instance, 3.5-month-old infants’ discrimination of dynamic emotional expressions in an intermodal matching task is enhanced when the infant's mother compared to a stranger is shown (Kahana-Kalman and Walker-Andrews, 2001, Montague and Walker-Andrews, 2002). However, to date no study has tested whether the effects of eye gaze cues on infants’ object processing are affected by familiarity of the face.
In the current study 4-month-old infants are presented with pictures of their caregiver (mother or father) and a stranger (another infant's mother or father) turning eye gaze either toward or away from a small object presented on the right or left side of the face. Then the objects are presented again without the face. We predict that 4-month-old infants will show an increased PSW response for non-cued objects compared to cued objects because cued objects have been more effectively encoded and require relatively less processing when being presented again without the face. This effect is expected to be stronger for the caregiver's face compared to a stranger's face. In addition, we predict a larger Nc amplitude in response to the caregiver's face compared to a stranger's face because this effect has been observed in previous research with 6-month-old infants (de Haan and Nelson, 1997, de Haan and Nelson, 1999).
2. Materials and methods
2.1. Participants
All participating infants were born full term (37–41 weeks) and were in the normal range for birth weight. Sixteen infants were included in the final sample (8 females, age range: 4 months, 2 days–4 months, 25 days; average age: 4 months and 13 days). Another 18 infants were tested but excluded from the sample because they failed to reach the minimum requirement of 10 artifact free trials per condition for averaging. This attrition rate can partly be accounted for by the relatively large number of four conditions tested within subjects, but it is within the typical attrition rate for infant ERP-studies of 50–75% (DeBoer et al., 2007). Two additional infants were excluded from the sample because their mothers were not photographed correctly prior to testing. Infants excluded from the final sample did not differ significantly from the included infants in terms of age (average age 4 months, 14 days) or sex ratio (8 females, 12 males; Mann–Whitney U-test, p < 0.3). All experiments were conducted with the understanding and informed consent of each participant's parent. The procedures of the study were approved by the ethics committee of the Fakultät für Verhaltens- und Empirische Kulturwissenschaften, Heidelberg.
2.2. Stimuli
The infant's mother (or in one case the father) was photographed in front of a light grey background (see Fig. 1 for an example). Caregivers were asked to look friendly, but calm, with no overt smiling. Three pictures were taken: one picture with eye gaze directed to the front, one picture with eye gaze averted to the left and one picture with eye gaze averted to the right. Caregivers were instructed to look toward the camera for the direct gaze picture and toward pre-defined positions in the room for the left and right averted gaze pictures. Caregivers were also asked not to move their heads when switching eye gaze between photographs. If necessary, several pictures were taken and caregivers received feedback to minimize head movement. The parent's clothes were covered with a black cape. Each parent served as the familiar face for his or her own infant and as a stranger for another participant. A father who accompanied a participating mother also had his picture taken and was only presented as the strange face for the one infant who came with his father. Caregivers and strangers were only matched for glasses (if they indicated that their infant most frequently sees them wearing glasses) and were otherwise dissimilar looking. Caregivers were asked whether they knew the stranger chosen for their infant prior to testing to ensure that infants were not familiar with the strangers. Portrait pictures were then overlaid with small pictures of colorful toys that were displayed next to the faces either to the left or right side, at the height of the pupils of the face. A number of 80 different objects were presented. Each object was presented once in the cued condition and once in the non-cued condition resulting in a maximum of 160 trials. Each object was presented only once in each half of the stimulus presentation. Faces were presented at a width of approximately 18 cm (SD = 2.8 cm, visual angle of 11.3°) and a height of 29 cm from head of hair to shoulder (SD = 1 cm, visual angle of 17.8°). Objects alone were about 7 cm × 7 cm of size (visual angle of 4°) and were presented at a distance of about 3 cm (visual angle of 2°) from the face at the height of the eyes. Luminance of the objects as measured with GIMP 2.6 (mean of brightness values across the image ranging from 0 to 255) was on average 193 (SD = 25). All objects were abstract toys.
Fig. 1.
Stimuli. Example of a mother who was presented as the familiar face to her own infant and as a strange face to another infant. In half of the trials the object was cued by the person's eye gaze and in half of the trials the object was non-cued. Gaze direction and object location were counterbalanced across trials.
2.3. Procedure
Infants sat on their caregiver's lap in a dimly lit room, at a viewing distance of 90 cm away from a 70 Hz 19-in. stimulus monitor. The experiment consisted of one block with 160 trials (40 trials per condition: cued/caregiver, non-cued/caregiver, cued/stranger, non-cued/stranger). Stimuli were presented using the software Presentation (Neurobehavioral Systems, Albany, USA). The four conditions were presented to the infant in a random order with the constraints that the same gaze condition (cued/non-cued) was not repeated more than 3 times consecutively and that the same familiarity condition (caregiver/stranger) was not repeated more than 3 times consecutively. Furthermore, object location and eye gaze direction were repeated 3 times maximum. Because of an error in the initial program, these restrictions were only applied in the first 52 trials for one of the subjects. After trial 52 for this one subject the non-cued condition was shown up to 6 times in a row and after trial 74 up to 7 cued trials were presented consecutively. Re-running all statistical analyses without this one participant did not yield any different effects, thus the infant was included in the final sample. Each trial started with a centrally presented face with gaze directed to the front and a small colorful object on the left or right side next to the face (Phase 1: caregiver or stranger, presented for 1000 ms), followed by the same face with gaze directed to the left or right side either toward the object or away from the object (Phase 2: 1500 ms), resulting in an apparent movement of the eyes from the front to the side as used in previous research on gaze motion processing (Watanabe et al., 2006). The face, directing gaze either toward or away from the object, was followed by a brief blank screen period (400–600 ms), and then the object was presented alone in the centre of the screen (Phase 3: 1000 ms). Each trial was followed by a blank screen period, whose duration varied randomly between 600 and 800 ms. If the infant became fussy or uninterested in the stimuli, the experimenter gave the infant a short break. The session ended when the infant's attention could no longer be attracted to the screen. EEG was recorded continuously and the behavior of the infants was also video-recorded throughout the session.
2.4. EEG recording and analyses
EEG was recorded with a 32 channels ActiCap system (Brain Products, Gilching, Germany) containing active electrodes based on Ag/AgCl sensors, which were attached to an elastic cap and mounted according to scalp locations of the 10–20 system. Data were amplified via a BrainAmp amplifier. Data were referenced to the right mastoid and recorded with a sampling rate of 250 Hz. Horizontal and vertical electro-oculograms were recorded bipolarly. EEG data were re-referenced offline to the linked mastoids and a bandpass filter was applied from 0.3 to 30 Hz. Artifacts caused by eye and body movements were removed from the data before averaging. In a first step, a gradient criterion was used for a semi-automatic artifact rejection allowing a maximum voltage step per sampling point of 100 μV to eliminate large movement artifacts. In addition, data were scanned manually trial per trial in order to match infants’ EEG data with the simultaneously video-recorded behavior and in order to detect small blinks and eye movements on EOG channels. Only trials were included in which the infant had looked to the screen during the whole trial (gaze to front, gaze to side, and object alone) and displayed no eye or body movements. ERPs were time-locked to the onset of the object alone (Phase 3). For additional analyses, ERPs were also averaged time-locked to the presentation of the face with gaze to the front and gaze to the side (Phases 1 and 2). Data were segmented into epochs from 200 ms before stimulus onset to 1500 ms after stimulus onset. A baseline correction was applied before averaging.
Each infant contributed 10–17 valid trials (mean of 12, SD 2) in the cued/caregiver condition, 10–19 valid trials (mean of 12, SD 3) in the non-cued/caregiver condition, 10–17 valid trials (mean of 11, SD 2) in the cued/stranger condition, and 10–16 valid trials (mean of 12, SD 2) in the non-cued/stranger condition.
3. Results
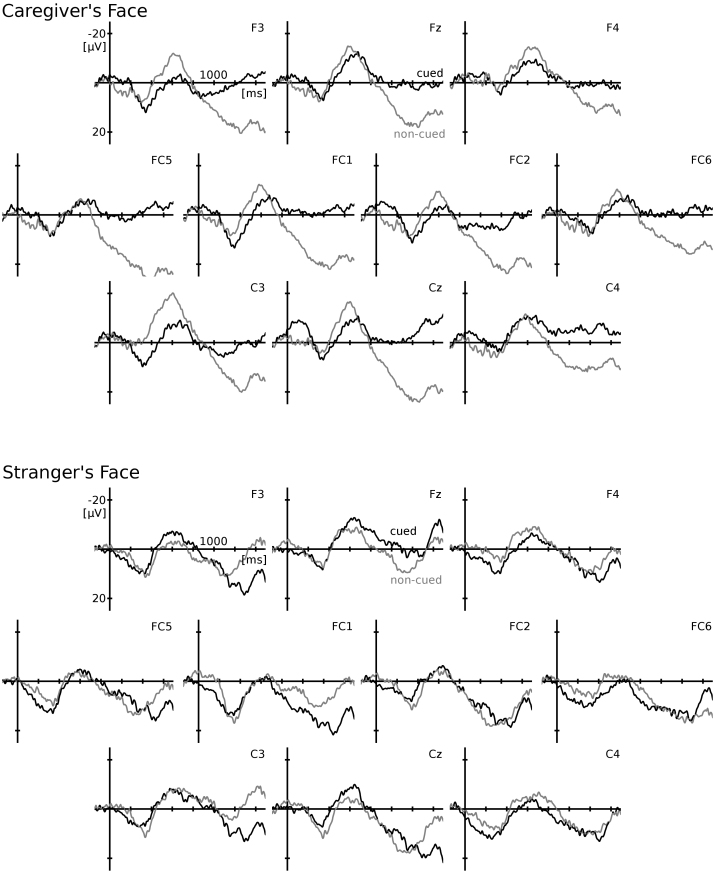
Grand average ERP responses for the cued and non-cued objects in the two familiarity conditions are presented in Fig. 2. On frontal and central channels a large negative deflection was observed in the mid-latency range: the Nc component which is typically evoked by visual stimulation in infants and whose amplitude has been associated with the amount of attention allocated toward a stimulus (Richards, 2003). Visual inspection suggested that there might be an effect of gaze condition on this component, thus amplitude was analyzed in the Nc time-window (400–800 ms). The Nc was followed by a positive slow wave response (PSW), which was particularly pronounced in the non-cued/caregiver condition and in the stranger conditions while waveforms returned to baseline following the Nc in the cued/caregiver condition. Amplitude of this slow wave was analyzed in a later time-window (1000–1500 ms). Greenhouse-Geisser corrections were employed where applicable in all reported statistical tests and level of significance was set at p < 0.05.
Fig. 2.
ERP results. Grand average ERP responses for the familiar face condition (upper panel) and the unfamiliar face condition (lower panel). When the caregiver's face was presented infants’ responses returned to baseline after the Nc for cued objects (black line) while a large PSW response was found in response to non-cued objects (grey line). When a stranger's face was presented a PSW was found for cued objects and non-cued objects which did not differ in amplitude across conditions. Note that negative is plotted upwards.
3.1. Negative central component
Mean amplitude between 400 and 800 ms after stimulus onset was taken as dependent variable in a repeated measures analysis of variance in order to assess differences in amplitude across conditions for the Nc. Within-subjects factors were familiarity (caregiver/stranger), gaze (cued/non-cued), and electrode (F3, Fz, F4, FC1, FC2, FC5, FC6, C3, Cz, C4). No significant main effects or interactions were found, all ps > 0.1. No effects were found when peak amplitude of the Nc was used for analysis instead of mean amplitude, all ps > 0.1. See Table 1 for means and standard deviations of Nc amplitude for all conditions.
Table 1.
Mean PSW and Nc amplitude in μV (PSW: 1000–1500 ms; Nc: 400–800 ms on frontal and central channels) and standard deviations (in parentheses) in response to the objects.
| PSW |
Nc |
|||
|---|---|---|---|---|
| Cued | Non-cued | Cued | Non-cued | |
| Caregiver | −0.81 (14.1) | 15.66 (12.3) | −3.76 (11.2) | −6.67 (14.9) |
| Stranger | 9.73 (16.9) | 6.99 (20.3) | −2.28 (14.4) | −2.28 (15.7) |
3.2. Positive slow wave
Mean amplitude was assessed in a time window between 1000 and 1500 ms after stimulus onset. The same statistical analyses were carried out as for the Nc. A significant main effect of gaze condition was found for amplitude of the PSW, F(1,15) = 5.24, p = 0.037, ηp2 = 0.259. Mean PSW amplitude was increased for objects in the non-cued condition (mean = 11.32 μV, SE = 2.8) compared to objects in the cued condition (mean = 4.45, SE = 3.3). There was also an interaction between familiarity and gaze condition, F(1,15) = 5.38, p = 0.035, ηp2 = 0.264. See Table 1 for means and standard deviations of PSW amplitude for all conditions.
When amplitude of the PSW was analyzed for the caregiver's face condition only, there was a highly significant main effect of gaze, F(1,15) = 17.5, p = 0.001, ηp2 = 0.539. Amplitude was larger for the non-cued objects (mean = 15.66 μV, SE = 3.1) compared to the cued objects (mean = −0.81 μV, SE = 3.5). When amplitude of the PSW was analyzed for the unfamiliar faces only, no main effect of gaze condition was found, F(1,15) = 0.2, p = 0.657, ηp2 = 0.013, and no interaction of electrode by gaze condition was found, F(9,7) = 0.87, p = 0.49, ηp2 = 0.055. This suggests that gaze condition only had an effect on infants’ object processing when their caregiver's face was presented.
When amplitude of the PSW was analyzed only for the cued objects, there was a significant main effect of familiarity, F(1,15) = 6.59, p = 0.021, ηp2 = 0.305. Amplitude was larger for objects cued by the stranger (mean = 9.73 μV, SE = 4.2) compared to objects cued by the caregiver (mean = −0.81 μV, SE = 3.5). There was also a significant interaction of familiarity by electrode, F(1,15) = 6.1, p = 0.013, ηp2 = 0.887. Subsequent t-tests contrasting amplitudes of both familiarity conditions for each electrode separately revealed that significant differences were found on FC1, FC6, and Cz, ps < 0.05 (two-tailed). Marginally significant differences were also observed on F3 and FC5, ps < 0.1 (two-tailed). On each of these channels amplitude was larger for cued objects in the stranger condition compared to cued objects in the caregiver condition suggesting that objects cued by the caregiver required less memory updating when being presented again compared to objects cued by a stranger which elicited a strong PSW response. When amplitude of the PSW was analyzed for the non-cued objects only, no main effect for familiarity condition was found F(1,15) = 1.92, p = 0.186, ηp2 = 0.113, and no interaction of electrode by familiarity condition was found, F(9,7) = 1.1, p = 0.481, ηp2 = 0.577, suggesting that non-cued objects were processed similarly in both familiarity conditions.
3.3. ERP responses to the faces
The PSW analyses showed significant differences in infants’ responses to the cued objects between both familiarity conditions. In order to examine whether caregivers’ and strangers’ faces were processed differently per se we also analyzed infants’ responses to the caregivers vs. strangers looking toward the front with the object next to the face (Phase 1 of each trial). In particular, an effect on the Nc component is conceivable as increased Nc amplitude was found for the mother's face compared to a stranger's face in previous research with 6-month-olds (de Haan and Nelson, 1997, de Haan and Nelson, 1999). Therefore, a repeated measures analysis was run with mean amplitude in the Nc time-window (400–800 ms) as dependent measure. Within-subjects factors were familiarity (caregiver/stranger) and electrode (F3, Fz, F4, FC1, FC2, FC5, FC6, C3, Cz, C4). Gaze was not included as an independent factor because in Phase 1 trials did not yet vary depending on the gaze condition. No main effect for familiarity condition, F(1,15) = 0.96, p = 0.343, ηp2 = 0.06, and no interaction of electrode by familiarity condition was found, F(9,7) = 1.02, p = 0.408, ηp2 = 0.064. Amplitude was similar for the caregivers’ faces (mean = −14.4 μV, SE = 2.8) and the strangers’ faces (mean = −17.8 μV, SE = 3.5).
We also analyzed ERP responses to faces looking to the side, either toward or away from the object (Phase 2 of each trial). No distinct positive or negative deflection was observed in response to stimuli in Phase 2 of the trial presentation. This is likely because there was no pause between faces looking toward the front and faces with eye gaze directed to the side. The lack of a blank screen before stimulus onset and the immediate repetition of almost identical face stimuli likely caused a suppression of ERP responses. For statistical analyses we thus chose a larger time-window based on visual inspection in which slight amplitude differences between conditions were observed across fronto-central channels: 300–1000 ms. A repeated measures analysis of variance was run on mean amplitude with familiarity (caregiver/stranger), gaze (cued/non-cued), and electrode (F3, Fz, F4, FC1, FC2, FC5, FC6, C3, Cz, C4) as within-subjects factors. There was no significant main effect of familiarity condition, F(1,15) = 2.67, p = 0.123, ηp2 = 0.151, no interaction of familiarity by gaze condition, F(1,15) = 2.86, p = 0.112, ηp2 = 0.16, and no other significant main effects or interactions, all ps < 0.2.
4. Discussion
We addressed the question whether eye gaze cues of a familiar face have stronger effects on 4-month-old infants’ object processing compared to a stranger's gaze. As predicted, we found an increased PSW response for objects that were not cued by the caregiver's eye gaze compared to objects that were previously gaze-cued. No effect was found for the unfamiliar faces. Our results summarized in Table 1 and Fig. 2 reveal that only objects cued by the caregiver elicited a return of the ERP response to baseline almost immediately following the Nc. When responses to cued objects were contrasted directly for both familiarity conditions, cued objects in the stranger condition elicited a significantly larger PSW response compared to objects cued by the caregiver. This indicates that objects cued by the caregiver required less memory updating compared to objects cued by a stranger because PSW amplitude has been associated with memory encoding in previous research (Nelson, 1994, Nelson and Collins, 1992, Snyder, 2010). The non-cued objects, in contrast, required more elaborate processing, regardless of the familiarity condition, as evidenced by a large PSW for non-cued objects in the caregiver condition and in the stranger condition.
In the unfamiliar face condition infants showed an Nc and subsequent PSW that did not differ in amplitude between the cued and non-cued objects. This lack of an effect of eye gaze was unexpected, since in the original study by Reid and colleagues (2004) only strange faces were shown to the infants. Nonetheless, the authors found an increased PSW for non-cued objects similar to the effect we found it in the familiar face condition. Procedural differences between our study and the original study may have impeded the effect of gaze cues in the strange face condition in the current experiment. First, we used an apparent motion paradigm subsequently presenting a face with direct gaze and the same face with averted gaze because static pictures were easier to control and to produce with the participating mothers and fathers in the lab prior to testing compared to filmed clips. Reid et al. (2004), in contrast, showed filmed footage of eye movement, which presumably produced more natural gaze shifts. Furthermore, each infant in the current study received a different pair of faces, which may have introduced additional variance compared to the original study. Finally, four conditions were tested within-subjects compared to only two conditions in the original study, resulting in a smaller number of available trials per condition (in the study by Reid et al., 2004, infants contributed a minimum number of 15 trials per condition).
Infants showed no difference in the PSW response for cued and non-cued objects in the strange face condition. However, a strong effect was found in the familiar face condition: infants responded with an increased PSW to non-cued objects compared to objects previously cued by their caregiver's eye gaze. Responses to objects that were gaze-cued by the caregiver returned to baseline following the Nc indicating that these objects were fully encoded. This finding supports the view that eye gaze facilitates young infants’ object processing by directing infants’ attention to gaze-cued stimuli. Why does the caregiver's face in particular have this effect? In the following we discuss several factors that might have made the caregiver's eye gaze particularly salient for the infant and/or easier to process when compared to the stranger's gaze:
-
(1)
Increased attention was directed to the caregiver's face.
-
(2)
Processing of the caregiver's face and eye gaze was facilitated because of increased perceptual familiarity.
-
(3)
Processing of the caregiver's eye gaze was facilitated or enhanced because of personal familiarity and previous interactions.
These possibilities are not mutually exclusive. It might well be that several factors worked in combination rendering the caregiver's eye gaze cues more effective than the stranger's cues in the current study.
First, differences in attention between conditions should be considered. It is conceivable that infants paid more attention to the caregiver's face compared to a strange face because the caregiver's face is a highly salient stimulus for young infants and because seeing the caregiver's face on a screen may be particularly unusual. In previous research 6-month-old infants responded with an enhanced Nc response to their mother's face compared to a stranger's face which may be interpreted as reflecting the allocation of more attention toward the mother's face (de Haan and Nelson, 1997, de Haan and Nelson, 1999). To test for a similar effect in the current study we also analyzed infants’ Nc responses time-locked to the onset of the faces looking toward the front at the beginning of the trial. Infants showed no differences in Nc amplitude for their caregiver's face compared to the stranger's face. No differences in ERP responses were found for the faces looking to the side either. Thus, although infants apparently distinguished between their caregiver and the stranger this was not reflected in their ERP responses to the faces themselves. A different paradigm was used than in the studies by de Haan and Nelson, 1997, de Haan and Nelson, 1999 and younger infants were tested which may explain the lack of a familiarity effect for the Nc. Though we cannot rule out that attention played a role in the current study, we found no evidence that infants directed more attention to stimuli in the familiar face condition per se. An interpretation of the PSW effect for non-cued vs. cued objects in the familiar face condition solely based on attention thus seems unlikely. However, there may have been differences in infants’ processing of the caregiver's face compared to the stranger's face that cannot be captured by recording ERPs, e.g. activation in subcortical pathways involved in face and emotion processing (Johnson, 2005).
Apart from attention differences between conditions other functional mechanisms are conceivable. One possibility is that a highly familiar face is easier to “decode” for infants enabling a more efficient use of social cues like eye gaze direction as proposed by the DAM (Hoehl et al., 2009, Reid and Striano, 2007). According to the DAM, a social agent is first detected based on salient perceptual features like the presence of eyes and/or biological motion. This obligatory processing step should not differ as a function of personal familiarity. In a second step the agent is identified, e.g. based on individual facial characteristics. This processing step was probably facilitated in the caregiver condition because of the perceptual familiarity of the caregiver's face. Possibly, rapid identification of the caregiver's face enhanced and/or sped up the subsequent processing stages, namely detection of the other person's attention focus in relation to the self (eye contact in Phase 1 of each trial) and in relation to something in the environment (i.e. cued vs. non-cued objects in Phase 2 of each trial). In contrast, facial identity processing may have been more difficult in the stranger condition. Consequently, infants were only able to use the very subtle eye gaze cues provided by the caregiver, which could not be processed in the stranger condition in the current study. In fact, contrasting a highly familiar face with a complete stranger may have accentuated the influence of processing stage 2 of the DAM in the current experiment because infants may have been particularly engaged in comparing the stranger's face to their caregiver's face, thus neglecting the stranger's eye gaze cues in relation to the objects.
In the classic face processing model by Bruce and Young face recognition was separated from analyses of facial expressions and speech movement analysis (Bruce and Young, 1986). Subsequent accounts on face processing have also stressed the cognitive and anatomical dissociation between facial identity recognition and the perception of changeable aspects of a face such as emotional expression and eye gaze, although interactions between those functions were not ruled out per se (Haxby et al., 2000). This view is supported, for instance, by evidence that familiarity with a face does not affect the judgement of facial expressions in healthy adults (Bruce, 1986). It should be noted, however, that infants’ discrimination of emotional expressions in an intermodal matching task is enhanced when a highly familiar face (i.e. the infant's mother) is presented compared an unfamiliar face (stranger), or a relatively less familiar face (the infant's father when the mother is the primary caregiver, see Montague and Walker-Andrews, 2002). More recently it has been suggested that instead of completely distinct pathways for processing facial identity and communicative social cues a multidimensional system may process both kinds of information with parts of this system being relatively more involved in the analysis of facial identity than in analyses of social cues and vice versa, allowing for mutual influences of different kinds of information provided by a face (Calder and Young, 2005). Interestingly, at least in adult females effects of gaze cueing are enhanced for personally familiar faces relative to unfamiliar faces (Deaner et al., 2007). The current study is the first to show enhanced effects of gaze cues on object processing for familiar faces compared to unfamiliar faces in infants. Our finding is in line with the suggestion that a familiar face may be easier to identify by an infant, consequently facilitating the processing of attentional cues provided by the face as proposed by the DAM (Hoehl et al., 2009, Reid and Striano, 2007).
In the current study faces of caregivers were contrasted with completely unfamiliar faces. Thus, we cannot rule out that aspects relating to the relationship between caregiver and infant, e.g. quality of attachment, rather than purely visual experience with the face can account for the observed effects. It is possible that infants were primarily occupied with processing the information conveyed by their caregiver's eye gaze in the current experiment, thus neglecting the information provided by the stranger. In fact, infants may have been “picking out” the objects cued by the caregiver. Consequently, objects in the strange face condition were less well encoded and elicited a PSW regardless of the stranger's gaze direction. In a between-subject design presenting only strangers to one group of infants we would predict the same pattern of results as found by Reid et al. (2004).
Even in adults greater gaze cueing effects have been found for personally familiar faces (Deaner et al., 2007), whereas it does not make a difference whether the same previously unfamiliar face is presented throughout hundreds of trials compared to a different face being shown in every trial of a gaze cueing experiment (Frischen and Tipper, 2004). It is possible that infants at 4 months of age have learned in numerous situations that their caregiver's eye gaze is informative and it might consequently bear a specific meaning for them. This interpretation, however, would hardly be consistent with the notion that gaze cueing effects in 4-month-olds and younger infants primarily reflect automatic attention shifts (Hoehl et al., 2009). Future studies should manipulate face familiarity in order to directly test how much visual experience with a face (with or without face-to-face interaction) is necessary for infants to be able to use an adult's gaze cues in the current paradigm.
Future studies may also consider developmental changes in infants’ responding to and interacting with their caregivers as compared to strangers. For instance, whereas 6-month-olds show an increased Nc to pictures of their mother compared to a stranger (de Haan and Nelson, 1997), the opposite response pattern is found in 3- to 4-year-old children (Dawson et al., 2002). When faced with an ambiguous toy infants at 12 months of age prefer to look at a strange experimenter compared to their mother and regulate their behavior in accordance with the experimenter's emotional cues (Stenberg and Hagekull, 2007). In a free play situation infants at 7 and 9 months of age coordinate attention toward a toy more frequently with a stranger compared to their mother (Striano and Bertin, 2005). A recent longitudinal study using eye tracking showed that a “stranger preference” in terms of following gaze shifts to objects occurs between 4 and 6 months of age (Gredebäck et al., 2010). Taken together, these findings suggest that infants older than those tested in the current study may in fact be more inclined to interact with and gain information from strangers compared to their caregivers in experimental contexts.
To conclude, 4-month-old infants’ processing of novel objects is facilitated by an adult's gaze cues, especially if the infant's caregiver is presented. The caregiver's eye gaze may be particularly salient and/or easier to process for young infants. Our results suggest that familiarity with a face enhances the processing of eye gaze cues in young infants. It remains to be examined in future research whether the personal relationship or purely perceptual familiarity is crucial for the effect.
Acknowledgements
This research was supported by a TransCoop grant (3.1 – TCVERL-DEU/1135099) awarded by the Alexander von Humboldt Foundation to S. Hoehl and T. Striano. We are grateful to the infants and parents who participated.
References
- Bruce V. Influences of familiarity on the processing of faces. Perception. 1986;15(4):387–397. doi: 10.1068/p150387. [DOI] [PubMed] [Google Scholar]
- Bruce V., Young A.W. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Bushnell I.W.R., Sai F., Mullin J.T. Neonatal recognition of the mother's face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- Calder A.J., Young A.W. Understanding the recognition of facial identity and facial expression. Nature Reviews Neuroscience. 2005;6(8):641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- Cleveland A., Schug M., Striano T. Joint attention and object learning in 5- and 7-month-old infants. Infant and Child Development. 2007;16:195–306. [Google Scholar]
- Cleveland A., Striano T. The effects of joint attention on object processing in 4- and 9-month-old infants. Infant Behavior and Development. 2007;30(3):499–504. doi: 10.1016/j.infbeh.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Dawson G., Carver L., Meltzoff A.N., Panagiotides H., McPartland J., Webb S.J. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Recognition of the mother's face by six-month-old infants: a neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Deaner R.O., Shepherd S.V., Platt M.L. Familiarity accentuates gaze cuing in women but not men. Biology Letters. 2007;3(1):64–67. doi: 10.1098/rsbl.2006.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T., Scott L.S., Nelson C.A. Methods for acquiring and analysing infant event-related potentials. In: de Haan M., editor. Infant EEG and Event-Related Potentials. Psychology Press; Hove, England: 2007. [Google Scholar]
- Frischen A., Tipper S.P. Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. Journal of Experimental Psychology: General. 2004;133(4):516–533. doi: 10.1037/0096-3445.133.4.516. [DOI] [PubMed] [Google Scholar]
- Gredebäck G., Fikke L., Melinder A. The development of joint visual attention: a longitudinal study of gaze following during interactions with mothers and strangers. Developmental Science. 2010;13(6):839–848. doi: 10.1111/j.1467-7687.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Reid V.M., Parise E., Handl A., Palumbo L., Striano T. Looking at eye gaze processing and its neural correlates in infancy – implications for social development and autism spectrum disorder. Child Development. 2009;80(4):968–985. doi: 10.1111/j.1467-8624.2009.01311.x. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Wiese L., Striano T. Young infants’ neural processing of objects is affected by eye gaze direction and emotional expression. PLoS ONE. 2008;3(6):e2389. doi: 10.1371/journal.pone.0002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H. Subcortical face processing. Nature Reviews Neuroscience. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kahana-Kalman R., Walker-Andrews A.S. The role of person familiarity in young infants’ perception of emotional expressions. Child Development. 2001;72:352–369. doi: 10.1111/1467-8624.00283. [DOI] [PubMed] [Google Scholar]
- Montague D.R., Walker-Andrews A.S. Mothers, fathers, and infants: the role of person familiarity and parental involvement in infants’ perception of emotion expressions. Child Development. 2002;73(5):1339–1352. doi: 10.1111/1467-8624.00475. [DOI] [PubMed] [Google Scholar]
- Nelson C.A. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G., Fischer K., editors. Human Behavior and the Developing Brain. Guilford Press; New York: 1994. pp. 269–313. [Google Scholar]
- Nelson C.A., Collins P.F. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19(1):105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Parise E., Reid V.M., Stets M., Striano T. Direct eye contact influences the neural processing of objects in 5-month-old infants. Social Neuroscience. 2008;3(2):141–150. doi: 10.1080/17470910701865458. [DOI] [PubMed] [Google Scholar]
- Reid V.M., Striano T. Adult gaze influences infant attention and object processing: implications for cognitive neuroscience. European Journal of Neuroscience. 2005;21(6):1763–1766. doi: 10.1111/j.1460-9568.2005.03986.x. [DOI] [PubMed] [Google Scholar]
- Reid V.M., Striano T. The directed attention model of infant social cognition. European Journal of Developmental Psychology. 2007;4(1):100–110. [Google Scholar]
- Reid V.M., Striano T., Kaufman J., Johnson M.H. Eye gaze cueing facilitates neural processing of objects in 4-month-old infants. Neuroreport. 2004;15(16):2553–2555. doi: 10.1097/00001756-200411150-00025. [DOI] [PubMed] [Google Scholar]
- Richards J.E. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6(3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K. Neural correlates of encoding predict infants’ memory in the paired-comparison procedure. Infancy. 2010;15(3):270–299. doi: 10.1111/j.1532-7078.2009.00015.x. [DOI] [PubMed] [Google Scholar]
- Stenberg G., Hagekull B. Infant looking behavior in ambiguous situations: social referencing or attachment behavior? Infancy. 2007;11(2):111–129. [Google Scholar]
- Striano T., Bertin E. Coordinated affect with mothers and strangers: a longitudinal analysis of joint engagement between 5 and 9 months of age. Cognition and Emotion. 2005;19(5):781–790. [Google Scholar]
- Striano T., Reid V.M., Hoehl S. Neural mechanisms of joint attention in infancy. European Journal of Neuroscience. 2006;23(10):2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- Theuring C., Gredebäck G., Hauf P. Object processing during a joint gaze following task. European Journal of Developmental Psychology. 2007;4(1):65–79. [Google Scholar]
- Watanabe S., Kakigi R., Miki K., Puce A. Human MT/V5 activity on viewing eye gaze changes in others: a magnetoencephalographic study. Brain Research. 2006;1092(1):152–160. doi: 10.1016/j.brainres.2006.03.091. [DOI] [PubMed] [Google Scholar]