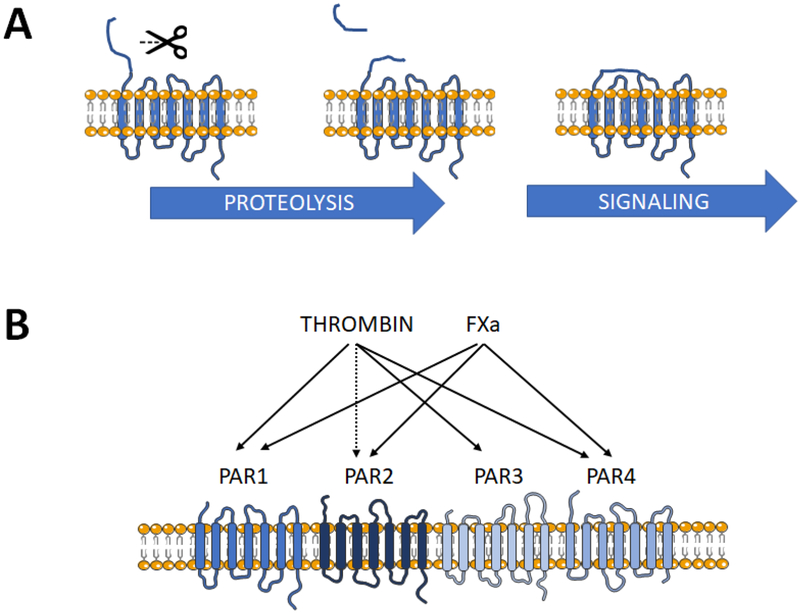
Fig. 2. Protease-activated receptors (PAR).
A) Simplified scheme depicting PAR activation. The N-terminal domain of the receptor is proteolytically cleaved at a specific recognition motif to generate a new N-terminus. The “tethered ligand” domain binds to and auto-activates the receptor, initiating G-protein-dependent signaling (possibly not in the case of PAR3). B) The relative selectivity of PAR for activation by thrombin and FXa. PAR1 is the predominant receptor for both coagulant proteases, PAR2 is potently activated by FXa and possibly by supraphysiological thrombin levels. PAR3 is cleaved by thrombin. PAR4 responds to thrombin (with 100-fold lower affinity than for PAR1) and modestly to FXa.