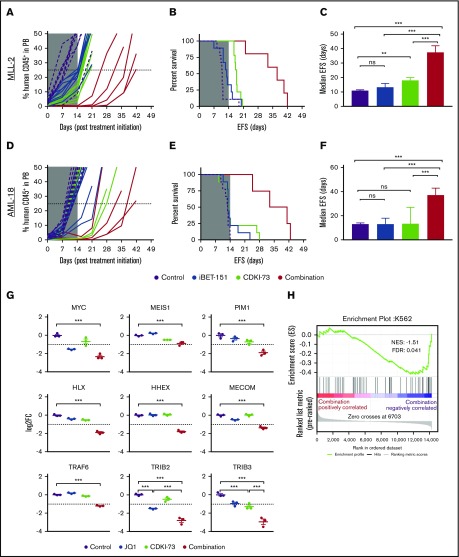
Figure 2.
In vivo efficacy of combined CDK9/BET inhibition against MLL-r ALL and AML PDXs. Human CD45+ cells (percentage of huCD45+) in the peripheral blood (PB) of engrafted mice (left panels; A,D), EFS curves (middle panels; B,E), and median EFS of engrafted recipients (right panels; C,F, error bars depict 95% confidence interval) for MLL-ALL (A-C, n = 9 per treatment group) and MLL-AML (D-F, n = 9 per treatment group). Mice were treated with vehicle (purple), iBET-151 (blue), CDKI-73 (light green), or the combination (red) for 14 days as indicated (gray shading indicates treatment duration). (G) Messenger RNA expression of selected genes measured by RNA-seq in AML-18 treated with CDKI-73, JQ1, or in combination for 4 hours (error bars depict standard error, Tukey multiple comparison test; **P < .01, ***P < .001; dashed line indicates log fold change (FC) less than or equal to −1 cutoff for negatively differentially expressed genes). (H) Gene-set enrichment analysis using the ranked gene-expression list as determined by RNA-seq for the comparison of combination treatment vs vehicle in AML-18 cells. Plot shows significant negative enrichment for transcription factor genes associated with superenhancers in K562 cells.22 No significant enrichment was observed for this gene set with ranked gene expression from single treatments (supplemental Table 4). FDR, false discovery rate; NES, normalized enrichment score; ns, not significant.