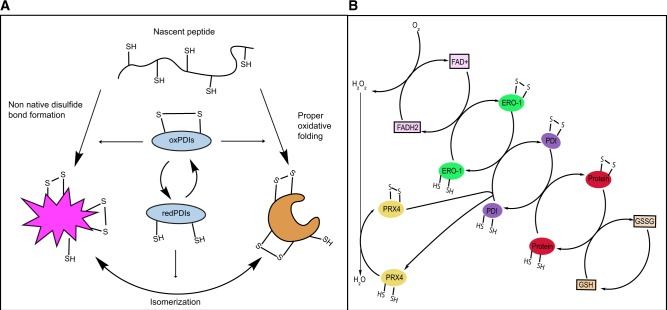
Fig. 2.
Functions of PDIs in oxidative folding. (A) Oxidized PDIs catalyse disulphide bond formation of nascent peptides in the ER. Leading to proper oxidative folding or non-native disulphide bond formation. Reduced PDIs facilitate isomerization of disulphide bonds. (B) PDIs are oxidized via interactions with ERO1. ERO1 uses FAD to transfer electrons to molecular oxygen generating hydrogen peroxide. PRX4 can also directly oxidize PDIs. PDIs transfer disulphides to client proteins. Glutathione contributes to disulphide bond reduction.