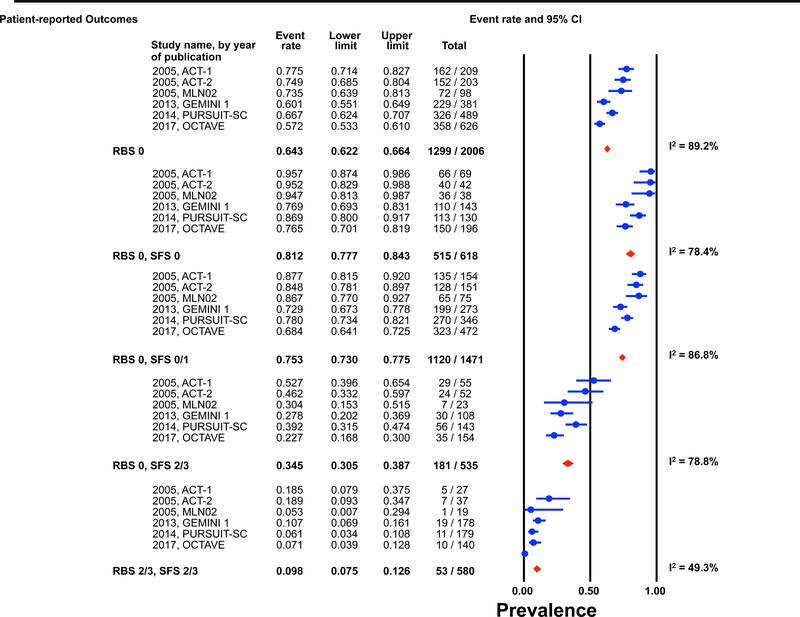
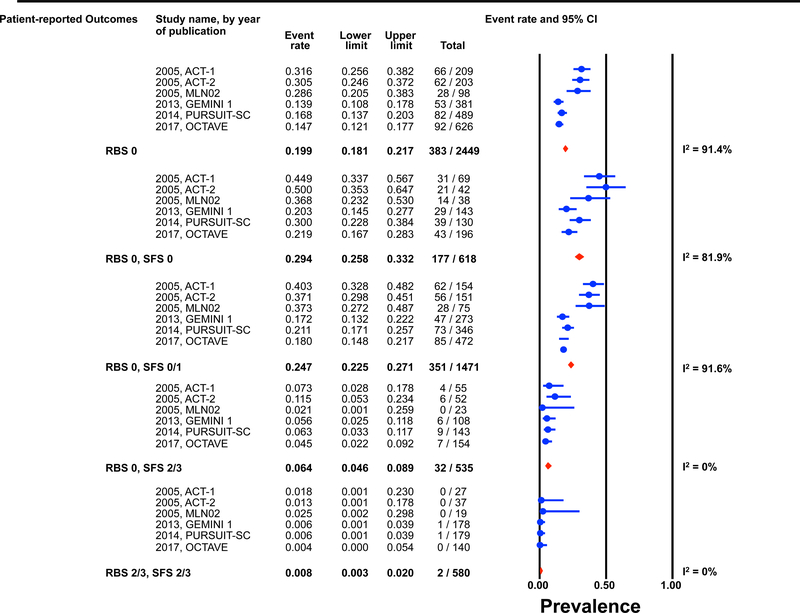
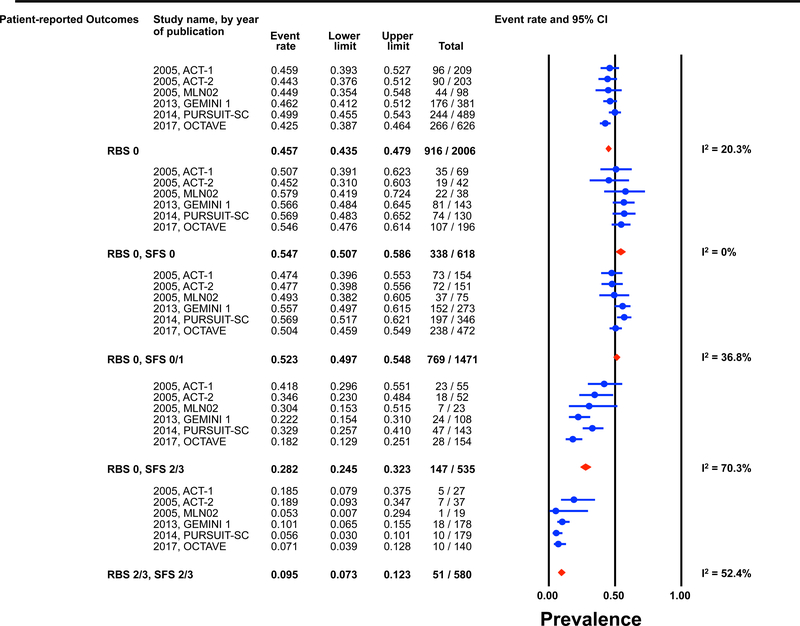
Figure 1: Pooled estimates from active intervention and placebo arms of ulcerative colitis clinical trials for prevalence of Mayo endoscopic scores post-induction by patient reported outcome permutations.
RBS: rectal bleedings core; SFS: stool frequency score; CI: Confidence interval Prevalence estimates are for the combined population of patients receiving active intervention (ie biologic or tofacitinib) or placebo. Individual study level estimates cannot be used to compare prevalence estimates across studies between different biologics or tofacitinib.
A: Endoscopic improvement (Mayo endoscopic score 0 or 1); B: Endoscopic remission (Mayo endoscopic scopre 0); C: Mild endoscopic activity (Mayo endoscopic score 1)