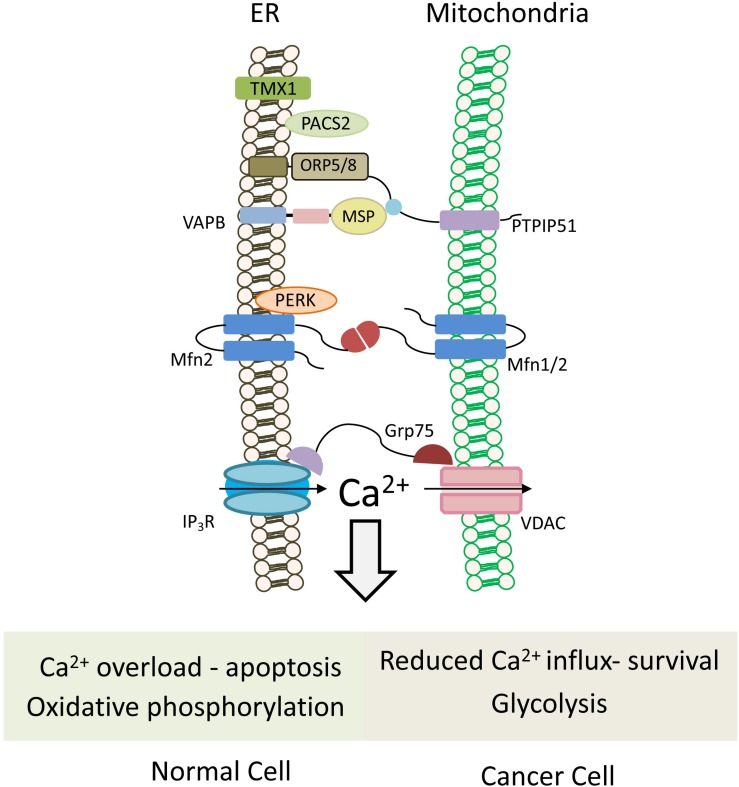
FIGURE 5.
Mitochondria-associated ER membranes in normal versus cancer cells. Schematic cartoon illustrating ER-mitochondria (MAMs) tethering proteins. MAMs regulate lipid transfer and play an important role in Ca2+ homeostasis by orchestrating Ca2+ shuttling from ER to mitochondria. Normal cells rely on oxidative phosphorylation for energy production, and possess normal MAM configuration, which promotes apoptotic cell death in response to calcium overloading. Conversely in cancer cells, which use the glycolytic pathway to produce ATP, expression level of tethering proteins is altered and “aberrant” MAMs are formed. In most cases, the ER-mitochondria contact is reduced and, hence, also the mitochondrial calcium uptake, favoring cell survival and resistance to chemotherapeutic drugs. Multiple proteins are involved in ER-mitochondria tethering (Sassano et al., 2017), those that are described in the text and the figures are: TMX1, thioredoxin related transmembrane protein 1; PTPIP51, protein tyrosine phosphatase-interacting protein 51; VAPB, VAMP-associated protein B; Mfn1/2, Mitofusin 1/2; PERK, protein kinase RNA-like ER kinase; GRP75, glucose-regulated protein 75; IP3R, IP3 (inositol 1,4,5-trisphosphate) receptor; VDAC, voltage-dependent anion channel; PACS2, phosphofurin acidic cluster sorting protein 2.