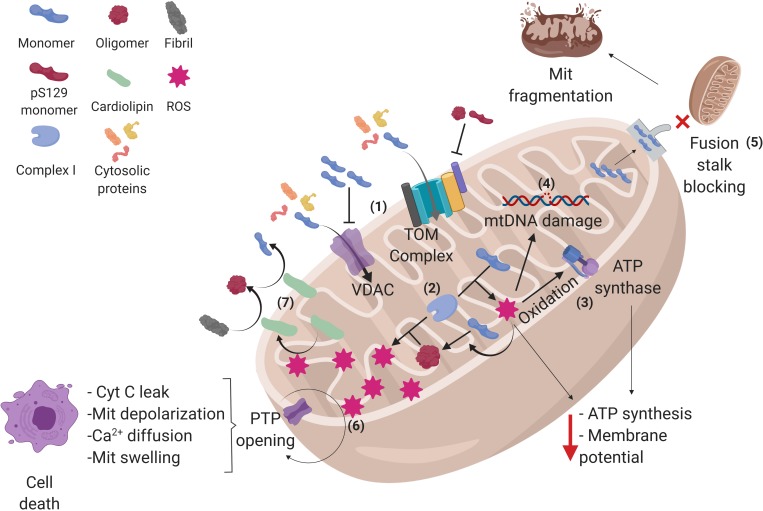
FIGURE 2.
Alpha-synuclein conformations and its interactions in mitochondria. (1) Alpha-synuclein (α-syn) monomers enter the mitochondria (Mit) through translocase of the outer membrane (TOM) and voltage-dependent anion channels (VDAC), but oligomers and pS129-α-syn on TOM and α-syn accumulation on VDAC can inhibit the protein import into the mitochondria, affecting the process that depends on cytosolic proteins. (2) Once inside, α-syn can interact with complex I of the electron transport chain, raising reactive oxygen species (ROS) production within the mitochondria, and favoring the aggregation of α-syn monomers into oligomers, which in turn produces more ROS, creating a cycle where α-syn aggregation and ROS production exacerbate each other. (3) The increased amount of ROS oxidizes the ATP synthase β-subunit, diminishing mitochondrial ATP levels as well as (4) damaging mitochondrial DNA (mtDNA), which may lead to an altered expression of mitochondrial genome-encoded genes. (5) α-syn monomers can also block mitochondrial fusion stalk, producing mitochondrial fragmentation. (6) In addition, α-syn induces the opening of permeability transition pore (PTP), allowing cytochrome C (cyt C) leak, depolarization, calcium diffusion, and swelling of the mitochondria, leading to cell apoptosis. (7) Certain stress events such as ROS can stimulate the translocation of cardiolipin from the inner membrane to the outer membrane, where it acts as a buffer for aggregative synuclein forms. See the text for further details. Created with BioRender.