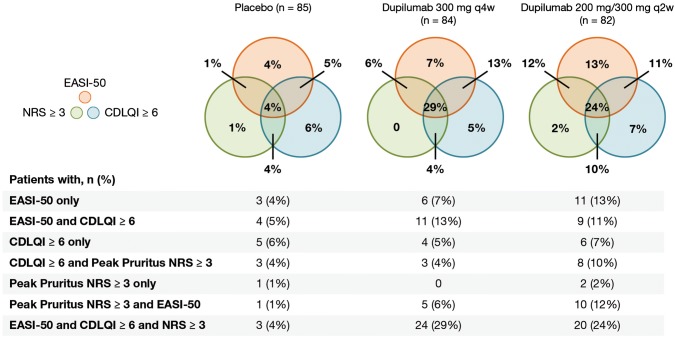
Fig. 2.
Proportions of patients in the full analysis set (FAS) who achieved clinically relevant thresholds for improvements in signs, symptoms, and/or quality of life (defined as 50% improvement from baseline in Eczema Area and Severity Index [EASI-50] or ≥ 3-point improvement from baseline in Peak Pruritus Numerical Rating Scale (NRS) score or ≥ 6-point improvement from baseline in Children’s Dermatology Life Quality Index [CDLQI]) at week 16. q2w every 2 weeks, q4w every 4 weeks