Table 1.
MAO inhibitory activity of AMPH derivatives and amiflamine analogues.
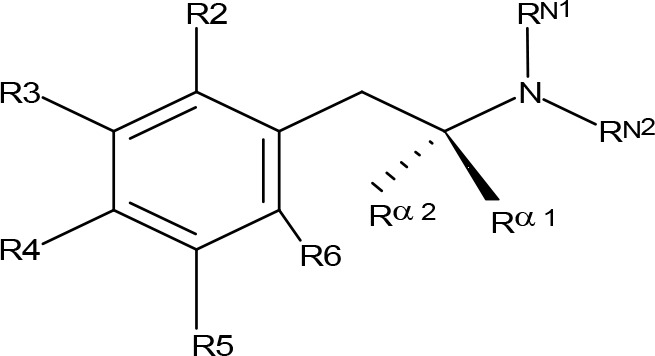 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAOI Activity IC50(Ki)a (µM) |
|||||||||||
| Compoundb | R2 | R3 | R4 | R5 | R6 | Rα1 | Rα2 | RN1 | RN2 | MAO-A | MAO-B |
| (+)-Amphetamine | H | H | H | H | H | CH3 | H | H | H | 20.0c;4.9d;33.8e | 770c;118d;161e |
| Amphetamine | H | H | H | H | H | HCH3 | H | H | 11.0f(5.3g) | 236g | |
| (-)-Amphetamine | H | H | H | H | H | H | CH3 | H | H | 70.0c;203e | 600c;180e |
| Methamphetamine | H | H | H | H | H | HCH3 | CH3 | H | 41h(17.2g) | > 200h(297)g | |
| Phentermine | H | H | H | H | H | CH3 | CH3 | H | H | 143i(88d;196g) | 285i(310d;138g) |
| AEPEA | H | H | H | H | H | HCH2CH3 | H | H | 14.0g | 234g | |
| N,α-DEPEA | H | H | H | H | H | HCH2CH3 | CH2CH3 | H | 251g | 159g | |
| Amiflamine/(+)-FLA336 | CH3 | H | N(CH3)2 | H | H | CH3 | H | H | H | 0.8j;2.0f | > 1000j |
| FLA336 | CH3 | H | N(CH3)2 | H | H | HCH3 | H | H | 2.7k | 440k | |
| (-)-FLA336 | CH3 | H | N(CH3)2 | H | H | H | CH3 | H | H | 3.0j | 125l |
| FLA289 | H | H | N(CH3)2 | H | H | HCH3 | H | H | 3.7l;2.0m | 400l | |
| FLA727 | H | H | NHCH3 | H | H | HCH3 | H | H | 0.55l-1.2m | 1500l | |
| (+)-FLA788 | CH3 | H | NHCH3 | H | H | CH3 | H | H | H | 0.13j | > 1000j |
| FLA558 | F | H | N(CH3)2 | H | H | HCH3 | H | H | 1.2k | 120k | |
| FLA314 | Cl | H | N(CH3)2 | H | H | HCH3 | H | H | 0.21k | 80k | |
| FLA405 | Br | H | N(CH3)2 | H | H | HCH3 | H | H | 0.22k | 100k | |
| FLA365 | Cl | H | N(CH3)2 | H | Cl | HCH3 | H | H | 0.013l | 180l | |
| FLA450 | Cl | H | N(CH3)2 | H | H | HCH2CH3 | H | H | 0.38k | 75k | |
| FLA463 | Cl | H | N(CH3)2 | H | H | CH3 | CH3 | H | H | 1.2k | 700k |
| FLA717 | CH3 | H | N(CH3)2 | H | H | CH3 | CH3 | H | H | 12.0k | 2100k |
| FLA384 | H | CH3 | N(CH3)2 | H | H | HCH3 | H | H | 8.0l | 650l | |
| (+)NBF003 | CH3 | H | N(CH3)2 | Br | H | CH3 | H | H | H | 1.1l | 480l |
aIC50 and/or Ki are reported, depending on the reference considered. bChemical name and/or common acronym and/or common name is given. When not indicated, the compound is the racemic mixture. c Mantle et al., 1976. d Ulus et al., 2000. e Robinson, 1985. f Scorza et al., 1997.g Santillo, 2014. h Matsumoto et al., 2014. i Kilpatrick et al., 2001. j Ask et al., 1982b. k Ask et al., 1982a. l Ask et al., 1985. m Reyes-Parada et al., 1994a.
