Table 2.
MAO inhibitory activity of AMPH derivatives monosubstituted in the aromatic ring.
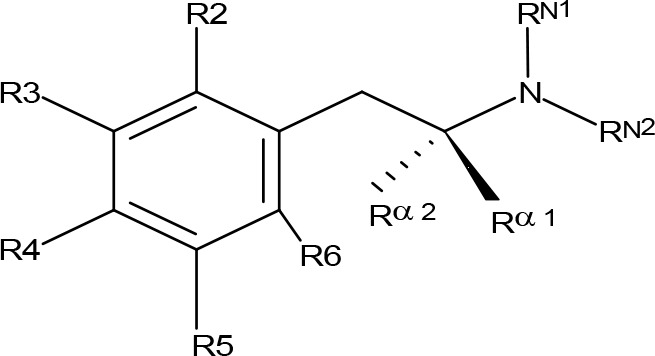 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAOI Activity IC50(Ki)a (µM) |
|||||||||||
| Compoundb | R2 | R3 | R4 | R5 | R6 | Rα1 | Rα2 | RN1 | RN2 | MAO-A | MAO-B |
| PMA/4-MeOA | H | H | OCH3 | H | H | HCH3 | H | H | 0.3c;0.6d(0.2e) | 45d(530e) | |
| 2-MeOA | OCH3 | H | H | H | H | HCH3 | H | H | 9.0e | 350e | |
| 3-MeOA | H | OCH3 | H | H | H | HCH3 | H | H | 23e | 1940e | |
| PMMA | H | H | OCH3 | H | H | HCH3 | CH3 | H | 1.7d | 58d | |
| 4-EtOA | H | H | OCH2CH3 | H | H | HCH3 | H | H | 0.22f | > 100f | |
| 4-PrOA | H | H | O(CH2)2CH3 | H | H | HCH3 | H | H | 0.13f | > 100f | |
| 4-BuOA | H | H | O(CH2)3CH3 | H | H | HCH3 | H | H | 0.32f | > 100f | |
| 4-BzOA | H | H | OCH2Phe | H | H | HCH3 | H | H | 3.42f | 0.71f | |
| MTA | H | H | SCH3 | H | H | HCH3 | H | H | 0.25g | NEg | |
| (+)-MTA | H | H | SCH3 | H | H | CH3 | H | H | H | 0.13h | NEh |
| (-)-MTA | H | H | SCH3 | H | H | H | CH3 | H | H | 2.04g | NEg |
| NMMTA | H | H | SCH3 | H | H | HCH3 | CH3 | H | 0.89g | NEg | |
| DMMTA | H | H | SCH3 | H | H | HCH3 | CH3 | CH3 | 2.10g | NEg | |
| NEMTA | H | H | SCH3 | H | H | HCH3 | CH2CH3 | H | 1.80g | NEg | |
| DEMTA | H | H | SCH3 | H | H | HCH3 | CH2CH3 | CH2CH3 | 6.45g | NEg | |
| NPMTA | H | H | SCH3 | H | H | HCH3 | (CH2)2CH3 | H | 2.41g | > 10g | |
| DPMTA | H | H | SCH3 | H | H | HCH3 | (CH2)2CH3 | (CH2)2CH3 | > 10g | NEg | |
| NBzMTA | H | H | SCH3 | H | H | HCH3 | CH2Phe | H | > 100f | > 100f | |
| MTAB | H | H | SCH3 | H | H | HCH2CH3 | H | H | 0.84g | NEg | |
| ETA | H | H | SCH2CH3 | H | H | HCH2CH3 | H | H | 0.10c | 29c | |
| (+)-ETA | H | H | SCH2CH3 | H | H | CH3 | H | H | H | 0.075h | > 100h |
| (+)-PTA | H | H | S(CH2)2CH3 | H | H | CH3 | H | H | H | 0.030h | 14.0h |
| ITA | H | H | SCH(CH3)2 | H | H | HCH3 | H | H | 0.40c | 8.1c | |
| (+)-BTA | H | H | S(CH2)3CH3 | H | H | HCH3 | H | H | 0.022h | 4.6h | |
| MSOA | H | H | SOCH3 | H | H | HCH3 | H | H | > 100i | NT | |
| MSO2A | H | H | SO2CH3 | H | H | HCH3 | H | H | > 100i | NT | |
| PCA/p-Chloroamphetamine | H | H | Cl | H | H | HCH3 | H | H | 4.0c;1.9j | NEc | |
| PBA/p-Bromoamphetamine | H | H | Br | H | H | HCH3 | H | H | 1.5j | NT | |
| PFA/p-Fluoroamphetamine | H | H | F | H | H | HCH3 | H | H | 16j | NT | |
| POHA | H | H | OH | H | H | HCH3 | H | H | 24.0k | NEk | |
| (+)-Fenfluramine | H | CF3 | H | H | H | CH3 | H | CH2CH3 | H | 256l | 800l |
| Fenfluramine | H | CF3 | H | H | H | HCH3 | CH2CH3 | H | 440m | 720m | |
| (-)-Fenfluramine | H | CF3 | H | H | H | H | CH3 | CH2CH3 | H | 115l | 685l |
| (+)-Norfenfluramine | H | CF3 | H | H | H | CH3 | H | H | H | 36l | 160l |
aIC50 and/or Ki are reported, depending on the reference considered. bChemical name and/or common acronym and/or common name is given. When not indicated the compound is the racemic mixture. c Scorza et al., 1997. d Matsumoto et al., 2014. e Green and el Hait, 1980. f Vilches-Herrera et al., 2009. g Hurtado-Guzmán et al., 2003. h Fierro et al., 2007. i Vallejos et al., 2002. j Fuller et al., 1975. k Arai et al., 1990. l Kilpatrick et al., 2001. m Leonardi and Azmitia, 1994. NE, No effect at 100 µM; NT, Not tested.
