Table 3.
MAO inhibitory activity of β-substituted AMPH derivatives.
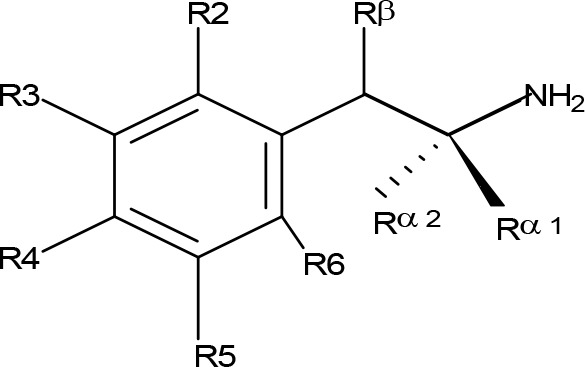 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAOI Activity IC50 a (µM) |
||||||||||
| Compoundb | Rβ | R2 | R3 | R4 | R5 | R6 | Rα1 | Rα2 | MAO-A | MAO-B |
| Cathinone | =O | H | H | H | H | H | HCH3 | NE | NE | |
| 4-MetOCat | =O | H | H | OCH3 | H | H | HCH3 | 77.0 | NE | |
| 4-EtOCat | =O | H | H | OCH2CH3 | H | H | HCH3 | 37.0 | > 100 | |
| 4-PropOCat | =O | H | H | O(CH2)2CH3 | H | H | HCH3 | 7.2 | 8.9 | |
| 4-ButOCat | =O | H | H | O(CH2)3CH3 | H | H | HCH3 | 14.4 | 6.0 | |
| (+)4-ButOCat | =O | H | H | O(CH2)3CH3 | H | H | CH3 | H | 29.5 | 5.6 |
| (-)4-ButOCat | =O | H | H | O(CH2)3CH3 | H | H | H | CH3 | 6.8 | 6.4 |
| 4-MetSCat | =O | H | H | SCH3 | H | H | HCH3 | 45.0 | > 100 | |
| (+)4-MetSCat | =O | H | H | SCH3 | H | H | CH3 | H | 44.5 | > 100 |
| (-)4-MetSCat | =O | H | H | SCH3 | H | H | H | CH3 | 38.9 | NT |
| 4-EtSCat | =O | H | H | S CH2CH3 | H | H | HCH3 | 15.1 | > 100 | |
| (+)4-EtSCat | =O | H | H | S CH2CH3 | H | H | CH3 | H | 12.9 | > 100 |
| (-)4-EtSCat | =O | H | H | S CH2CH3 | H | H | H | CH3 | 38.0 | NT |
| 4-MetONEPhe | OH | H | H | OCH3 | H | H | HCH3 | 9.8 | NE | |
| 4-EtONEPhe | OH | H | H | O CH2CH3 | H | H | HCH3 | 7.0 | NE | |
| 4-PropONEPhe | OH | H | H | O (CH2)2CH3 | H | H | HCH3 | 2.8 | 100 | |
| 4-ButONEPhe | OH | H | H | O (CH2)3CH3 | H | H | HCH3 | 4.7 | 65 | |
| 4-OHNEPhe | OH | H | H | OH | H | H | HCH3 | 220.0c | NEc | |
| 4-MetSNEPhe | OH | H | H | SCH3 | H | H | HCH3 | 7.3 | NE | |
| 4-EtSNEPhe | OH | H | H | S CH2CH3 | H | H | HCH3 | 1.9 | > 100 | |
| 4-PropSNEPhe | OH | H | H | S (CH2)2CH3 | H | H | HCH3 | 1.7 | > 100 | |
| BMetOA | OCH3 | H | H | H | H | H | HCH3 | > 100 | > 100 | |
| B,4DMetOA | OCH3 | H | H | OCH3 | H | H | HCH3 | 77.5 | > 100 | |
| BMetSA | OCH3 | H | H | SCH3 | H | H | HCH3 | 50.6 | > 100 | |
aUnless stated, IC50 values are from Osorio-Olivares et al., 2004. bChemical name and/or common acronym and/or common name is given. When not indicated the compound is the racemic mixture. c Arai et al., 1990. NE, No effect at 100 µM; NT, Not tested.
