Table 4.
MAO inhibitory activity of AMPH derivatives polysubstituted in the aromatic ring.
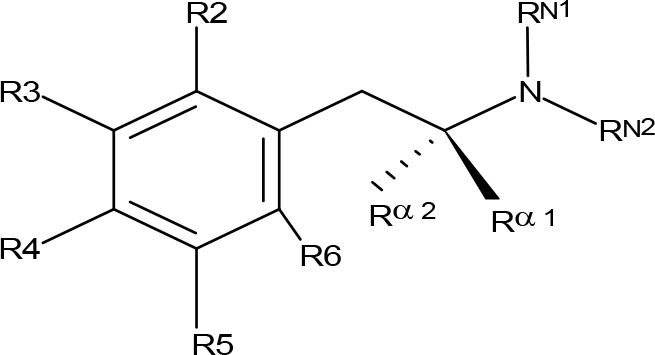 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAOI Activity IC50(Ki)a (µM) |
||||||||||||
| Compoundb | R2 | R3 | R4 | R5 | R6 | Rα1 | Rα2 | RN1 | RN2 | MAO-A | MAO-B | |
| 2,4-DMA | OCH3 | H | OCH3 | H | H | HCH3 | H | H | 0.6c | NEc | ||
| 3,4-DMA | H | OCH3 | OCH3 | H | H | HCH3 | H | H | 20c | NEc | ||
| 2,5-DMA | OCH3 | H | H | OCH3 | H | HCH3 | H | H | > 100f | NEf | ||
| 3,4,5-TMA | H | OCH3 | OCH3 | OCH3 | H | HCH3 | H | H | NEc;NId | NEc;NId | ||
| 2,4,5-TMA | OCH3 | H | OCH3 | OCH3 | H | HCH3 | H | H | NEc | NEc | ||
| 2,4,6-TMA | OCH3 | H | OCH3 | H | OCH3 | HCH3 | H | H | 0.4e | NEe | ||
| 2-Br-DMA | Br | H | OCH3 | OCH3 | H | HCH3 | H | H | 9.3c | NEc | ||
| 5-Br-DMA | OCH3 | H | OCH3 | Br | H | HCH3 | H | H | 13.0c | NEc | ||
| 2-NO2-DMA | NO2 | H | OCH3 | OCH3 | H | HCH3 | H | H | NEc | NEc | ||
| 6-Cl-DMA | OCH3 | H | OCH3 | H | Cl | HCH3 | H | H | 0.07e | NEe | ||
| ALEPH-1 | OCH3 | H | SCH3 | OCH3 | OCH3 | HCH3 | H | H | 5.1c | NEc | ||
| ALEPH-2 | OCH3 | H | SCH2CH3 | OCH3 | H | HCH3 | H | H | 3.2c | NEc | ||
| 4-PrS-DMA | OCH3 | H | S(CH2)2CH3 | OCH3 | H | HCH3 | H | H | 2.4e | NEe | ||
| 4-BuS-DMA | OCH3 | H | S(CH2)3CH3 | OCH3 | H | HCH3 | H | H | 2.9e | NEe | ||
| 4-PentS-DMA | OCH3 | H | S(CH2)4CH3 | OCH3 | H | HCH3 | H | H | 14.3e | NEe | ||
| 2,5-DM-MTAB | OCH3 | H | SCH3 | OCH3 | H | HCH2CH3 | H | H | 30.9e | NEe | ||
| 2,5-DM-ETAB | OCH3 | H | SCH2CH3 | OCH3 | H | HCH2CH3 | H | H | 11.8e | NEe | ||
| 2,6-DM-MTA | OCH3 | H | SCH3 | H | OCH3 | HCH3 | H | H | 0.30e | NEe | ||
| 2,6-DM-ETA | OCH3 | H | SCH2CH3 | H | OCH3 | HCH3 | H | H | 0.08e | NEe | ||
| 4-ESO-2,5-DMA | OCH3 | H | SOCH3 | OCH3 | H | HCH3 | H | H | > 100f | NT | ||
| 4-ESO2-2,5-DMA | OCH3 | H | SO2CH3 | OCH3 | H | HCH3 | H | H | NEf | NT | ||
| DOM | OCH3 | H | CH3 | OCH3 | H | HCH3 | H | H | 24.0c | NEc | ||
| DOI | OCH3 | H | I | OCH3 | H | HCH3 | H | H | 24c;37d | NEc | ||
| DOB | OCH3 | H | Br | OCH3 | H | HCH3 | H | H | 100c | NEc | ||
| DON | OCH3 | H | NO2 | OCH3 | H | HCH3 | H | H | NEc | NEc | ||
| DOTFM | OCH3 | H | CF3 | OCH3 | H | HCH3 | H | H | NEc | NEc | ||
| MDA | H | CH2-O-CH2 | H | H | HCH3 | H | H | 9.3c(8.5g) | NEc | |||
| 2Br-MDA | Br | H | CH2-O-CH2 | H | HCH3 | H | H | 13.0c | 64.0c | |||
| 2Cl-MDA | Cl | H | CH2-O-CH2 | H | HCH3 | H | H | 6.3c | 38.0c | |||
| 2NO2-MDA | NO2 | H | CH2-O-CH2 | H | HCH3 | H | H | NEc | NEc | |||
| MDMA | H | CH2-O-CH2 | H | H | HCH3 | CH3 | H | 30c(24.7g) | NEc | |||
| (+)-MDMA | H | CH2-O-CH2 | H | H | CH3 | H | CH3 | H | 44h(22h) | 370h | ||
| (-)-MDMA | H | CH2-O-CH2 | H | H | H | CH3 | CH3 | H | 56h(28.3h) | 378h | ||
aIC50 and/or Ki are reported, depending on the reference considered. bChemical name and/or common acronym and/or common name is given. When not indicated the compound is the racemic mixture. c Scorza et al., 1997. d Matsumoto et al., 2014. e Gallardo-Godoy et al., 2005. f Vallejos et al., 2002. g Steuer et al., 2016. h Leonardi and Azmitia, 1994. NE, No effect at 100 µM; NI, No inhibition at 200 µM; NT, Not tested.
