Table 5.
MAO inhibitory activity of some AMPH derivatives containing aromatic systems larger than benzene.
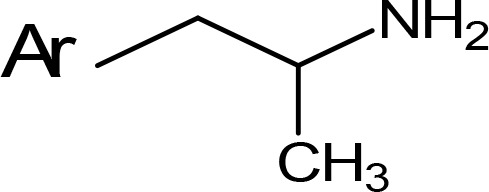
| |||
|---|---|---|---|
| MAOI Activity IC50(Ki)a (µM) |
|||
| Compoundb | Ar | MAO-A | MAO-B |
| NIPA/PAL-287 |
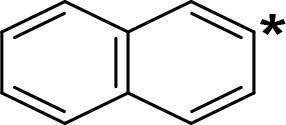
|
0.42c | > 100c |
| 6-MeO-NIPA |
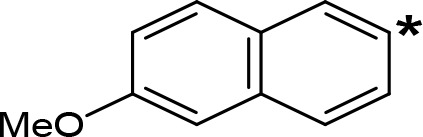
|
0.18c | 16.3c |
| 6-EtO-NIPA |
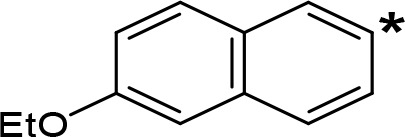
|
0.45c | 13.6c |
| 6-PrO-NIPA |
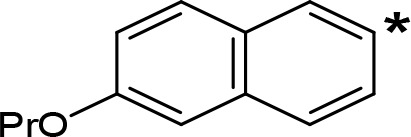
|
0.68c | 13.5c |
| 6-BuO-NIPA |
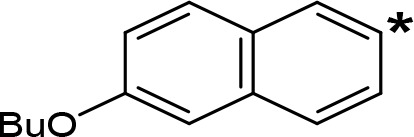
|
1.53c | NT |
| 6-MeS-NIPA |
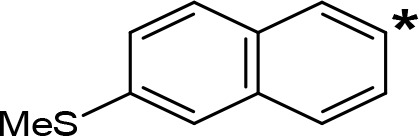
|
0.50c | NT |
| 2-Benzofuryl-IPA |
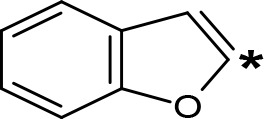
|
0.80d | > 100d |
| AMT |
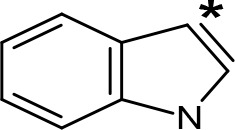
|
0.38e | > 10e |
| 4-MeO-AMT |
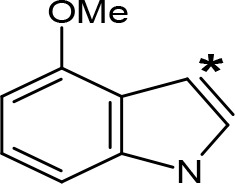
|
1.4e | > 10e |
| 5-MeO-AMT |
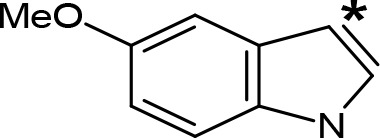
|
31e | > 10e |
| 5-Me-AMT |
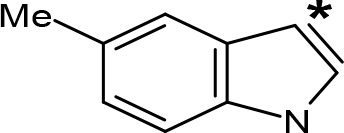
|
1.5e | > 10e |
| 5-F-AMT |
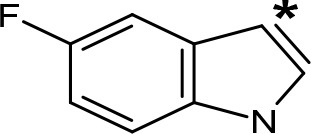
|
0.45e(0.032f) | 376e(575f) |
| 5-Cl-AMT |
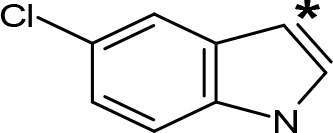
|
0.25e | 82e |
| 7-Me-AMT |
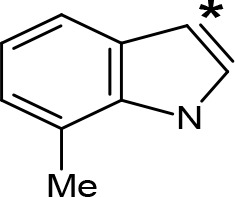
|
0.049e | > 10e |
aIC50 and/or Ki are reported, depending on the reference considered. bChemical name and/or common acronym and/or common name is given. c Vilches-Herrera et al., 2009. d Vallejos et al., 2005. e Wagmann et al., 2017. f Kinemuchi et al., 1988. NT, Not tested. Ar, Aromatic ring. *: This symbol denotes where, in the aromatic ring, the aliphatic side chain is linked. Me, Methyl; Et, Ethyl; Pr, Propyl; Bu, Butyl.
