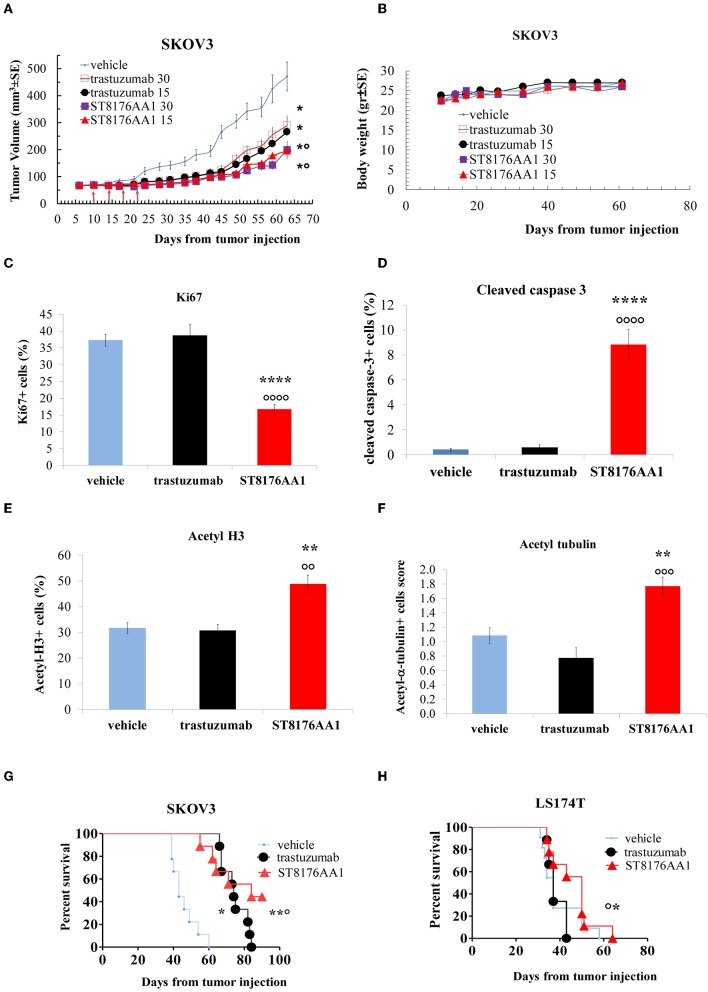
Figure 8.
Anti-tumor efficacy of ST8176AA1 in tumor xenografts. (A) Subcutaneous tumors were induced in nude mice (10/group) by injecting human SKOV3 cells ovary carcinoma. When tumor masses reached an average size of 50 mm3 mice were treated i.p. (4 doses of 15 or 30 mg/kg once every 4 days, starting 10 days after tumor cell transplantation) with ST8176AA1 or trastuzumab. One group received vehicle (PBS) with the same schedule. Tumor growth was monitored using a Vernier caliper. (B) Body weight. Data are the mean ± SE. Statistical analysis by Mann-Whitney's test *P < 0.05 vs. vehicle and °P < 0.05 vs. trastuzumab; (C–F) Tumor masses at the end of study in (A) were analyzed by immunohistochemistry. Cells positive for Ki67 (C), cleaved-caspase 3 (D), acetyl-H3 (E), acetyl-α-tubulin (F) were counted by two independent observers in five randomly selected fields. Data in the graph are expressed as the mean of positive cells × 100/total cells ± SE or as score with negative staining, score 0; 1-20% positive cells, score 1+; 21-50% positive cells, score 2+ >50% positive cells, score 3+. Statistical analysis by Mann-Whitney's test. **P < 0.01 and ****P < 0.0001 vs. vehicle-treated group; °°P < 0.01, °°°P < 0.001, and °°°°P < 0.0001 vs. trastuzumab. (G) Orthotopic tumor models of ovarian and colon (H) cancer by tumor cell injection in the peritoneum of mice (10/group). Mice were treated ip with ST8176AA1 or trastuzumab (4 doses of 15 mg/kg once every 4 days, starting 3 days after tumor cell transplantation). One group received vehicle (PBS) with the same schedule. Median survival time and Kaplan Myers were evaluated. Data are expressed as mean ± SE, *P < 0.05 and **P < 0.01 vs. vehicle and °P < 0.05 vs. trastuzumab.