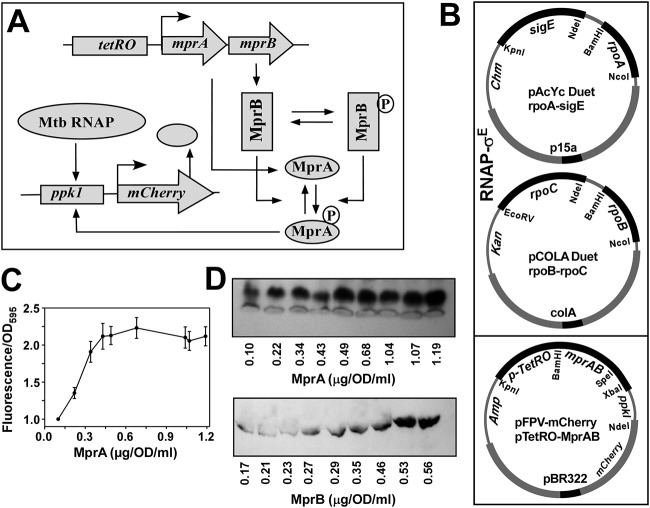
FIG 1.
In vivo recombinant reporter assay for assessing the MprAB output as a function of increasing concentration of both MprA and MprB. (A) Schematic diagram of tuning and functioning of the MprAB TCS circuit, in which the MprA and MprB proteins can be systematically controlled by the tetRO-inducible promoter. The MprB protein subsequently phosphorylates and dephosphorylates MprA to produce an MprA∼P pool, which in turn induces the mCherry gene through the ppk1 promoter. The output of the circuit (mCherry fluorescence) can be monitored as a function of the tetRO inducer anhydrotetracycline (ATC). Mtb, M. tuberculosis. (B) Design of the synthetic circuit using a three-plasmid reporter system in E. coli composed of pAcYc Duet-rpoA and sigE, and pCOLA Duet-rpoB-rpoC for the expression of the M. tuberculosis RNAP-σE holoenzyme, pFPV mCherry with the tetRO-inducible mprAB genes, and the mCherry reporter gene under the control of the MprA∼P-inducible ppk1promoter. Origins of replication and antibiotic resistances of each plasmid are highlighted. (C) Relative fluorescence intensities of mCherry expression with respect to control (no inducer) for OD 1 cells (average from 3 replicates with standard deviation) were plotted against various concentrations of MprA (average from 3 replicates) induced by 0, 10, 20, 40, 80, 160, 200, 240, and 320 ng/ml of anhydrotetracycline. (D) Representative Western blot analysis of OD 1 cells from each point of induction against respective antibodies of MprB and MprA and corresponding protein concentration are shown.