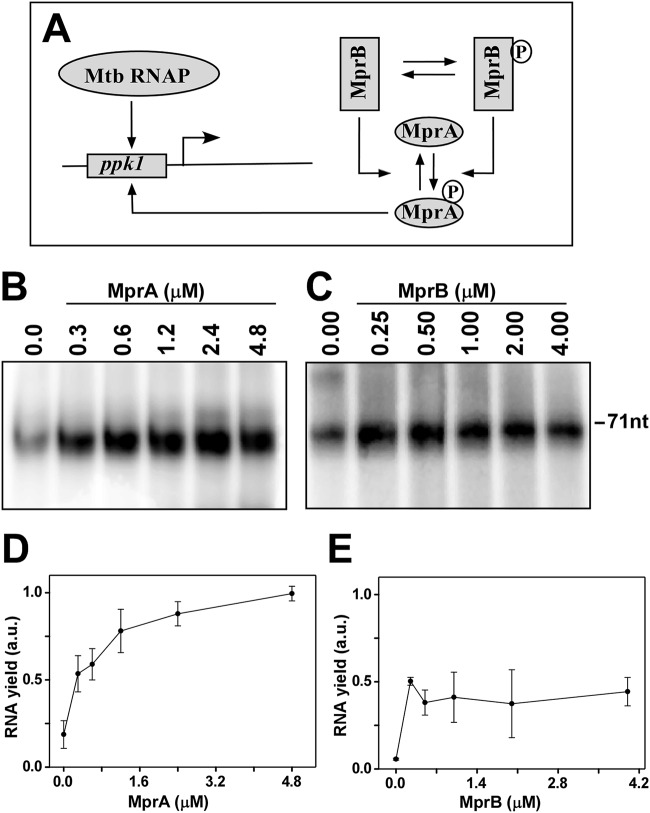
FIG 4.
In vitro transcription assays using radiolabeled [α-32P]CTP. (A) Schematic diagram of functioning of the MprAB TCS circuit in the transcription assay. Mtb, M. tuberculosis. (B) M. tuberculosis RNAP core (0.4 μM) and 0.8 μM σE were incubated with 0.1 μM ppkI promoter DNA and various concentrations of phosphorylated MprA as in the reaction in Fig. 3A (0.3, 0.6, 1.2, 2.4, and 4.8 μM while keeping MprB fixed at 1.0 μM). Transcription reactions were initiated by adding NTP mix (250 μM ATP, GTP, UTP, and 20 μM CTP containing 0.4 μCi [α-32P]CTP) and heparin (25 μg/ml) at 37°C for 30 min and terminated using formamide dye. Reaction products were kept for 2 min at 95°C, run on a 12.5% urea-PAGE gel, and scanned in a phosphorimage scanner. Transcript size was 71 nt. (C) Assay repeated as panel B but MprA∼P was added from the reaction as in panel B (keeping MprA fixed at 0.50 μM with 0.25, 0.5, 1.0, 2.0, and 4.0 μM MprB). (D and E) Average relative transcript yield (with respect to RNAP holosample in the presence of either MprA or MprB) from 3 replicates with standard error are plotted against concentrations of MprA and MprB, respectively.