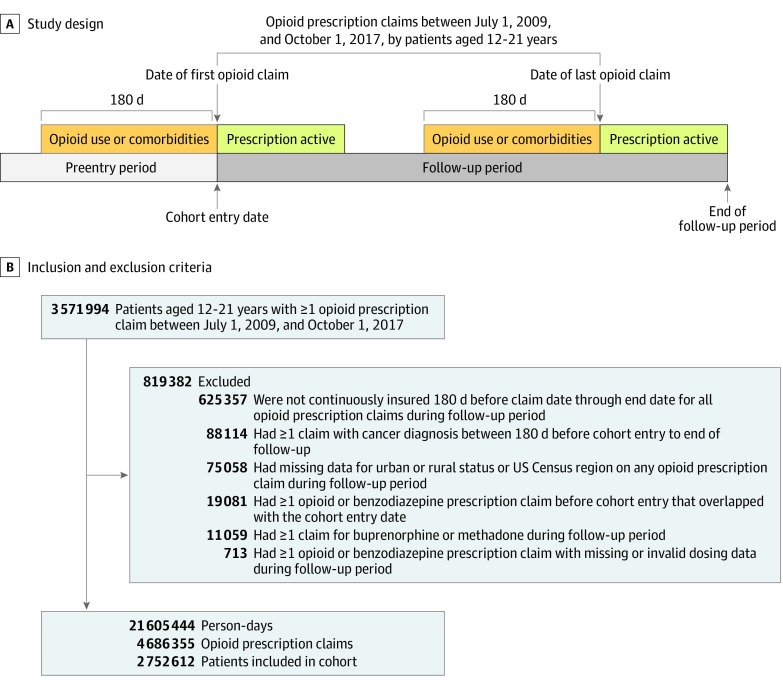
Figure 1. Study Design and Sample Inclusion and Exclusion Criteria.
Study design for a patient with 2 opioid prescription claims. For a patient with only 1 claim, follow-up terminated at the end of the active period of this claim. Recent opioid use was defined based on the presence of an opioid prescription claim during the 180 days to 1 day before the date of the opioid prescription claim from which person-days derived. Comorbidities were defined based on the presence of diagnosis codes on claims that occurred on the date of each opioid prescription claim or during the 180 days before this date.