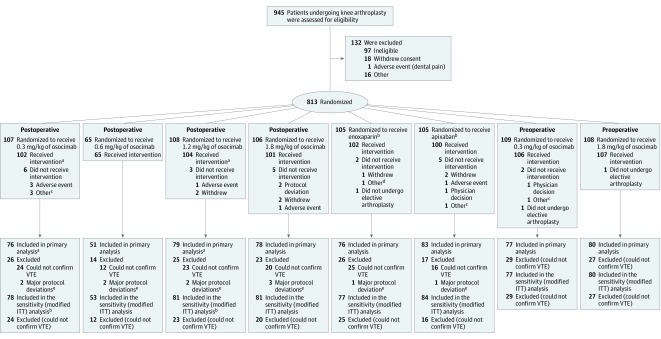
Figure. Enrollment, Randomization, and Populations for Analysis in the FOXTROT Trial of Osocimab to Prevent Venous Thromboembolism (VTE).
aA patient assigned to receive osocimab 1.2 mg/kg postoperatively received 0.3 mg/kg.
bOf these patients, 62 and 43 were randomized as part of the postoperative and preoperative osocimab phases.
cRescheduled or canceled knee arthroplasty.
dReceived commercial enoxaparin instead of study drug.
eDeviations from inclusion or exclusion criteria, use of prohibited concomitant medications impacting on the primary outcome, and significant changes to study drug intake were considered major protocol deviations.