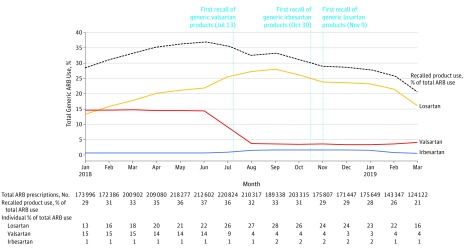
Figure 2. Utilization Trends for Generic ARB Products Affected by Recalls Between January 2018 and March 2019.
The dashed line indicates utilization of products affected by recalls as a proportion of total generic angiotensin receptor blocker (ARB) utilization. The vertical lines indicate calendar dates of the first product recalls of valsartan, irbesartan, and losartan. Total numbers of prescriptions filled for all products affected by recalls every month are reported in the first row beneath the x-axis; percentages of total generic ARB use accounted for by recalled valsartan, irbesartan, and losartan products are also shown.