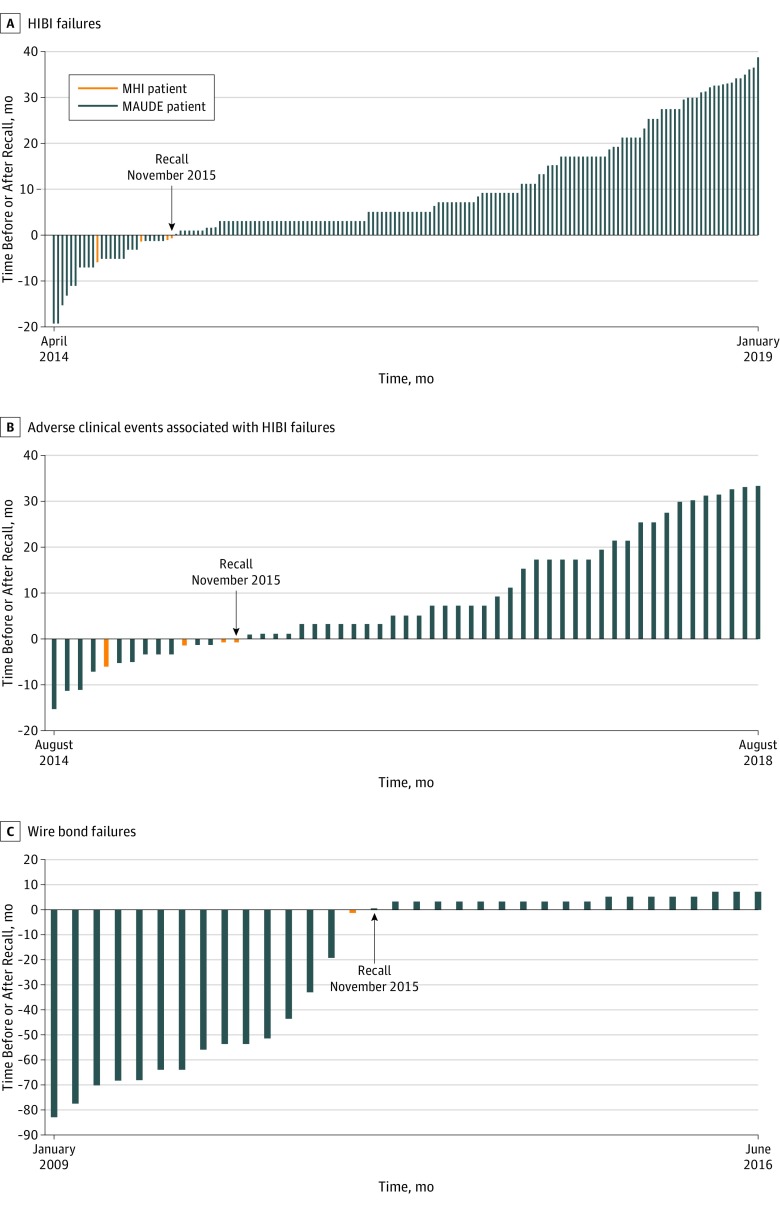
Figure 2. Timeline for Device Failures and Adverse Events Before and After Recall of the Pacemaker on November 9, 2015.
A, Each vertical bar represents 1 device found to have high internal battery impedance (HIBI). B, Each vertical bar represents 1 serious adverse clinical event associated with a device HIBI failure. C, Each vertical bar represents a device failure owing to lifted bond wires; 3 adverse events were associated with this defect and all occurred prior to the recall.
MAUDE indicates Manufacturer and User Facility Device Experience database; MHI, Minneapolis Heart Institute.