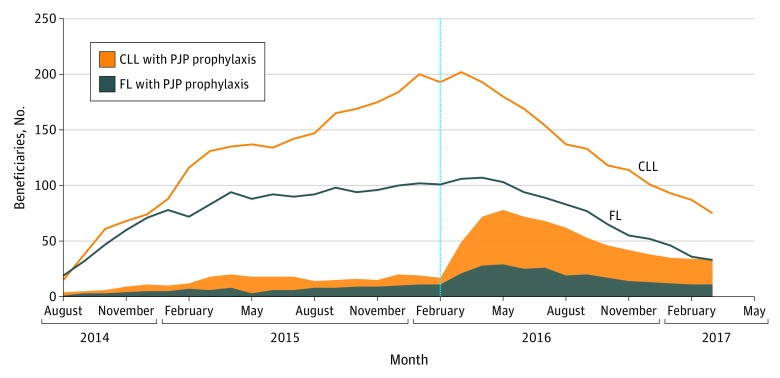
Figure 2. Number of Study Medicare Beneficiaries Receiving IDEL and PJP Prophylaxis by Indication and Calendar Month.
CLL indicates chronic lymphocytic leukemia; FL, follicular lymphoma; IDEL, idelalisib; PJP, Pneumocystis jirovecii pneumonia. IDEL-treated beneficiaries were identified from July 2014 (approval) through September 2016 and were followed through March 2017. Note that the first prescription that fulfilled all study entry criteria did not occur until August 2014. PJP prophylaxis was defined as on-treatment receipt of trimethoprim-sulfamethoxazole, pentamidine, atovaquone, dapsone, or clindamycin. The vertical dashed line represents the timing of the safety communications on risk for serious infections with idelalisib and the recommendation for PJP prophylaxis in all IDEL-treated patients.