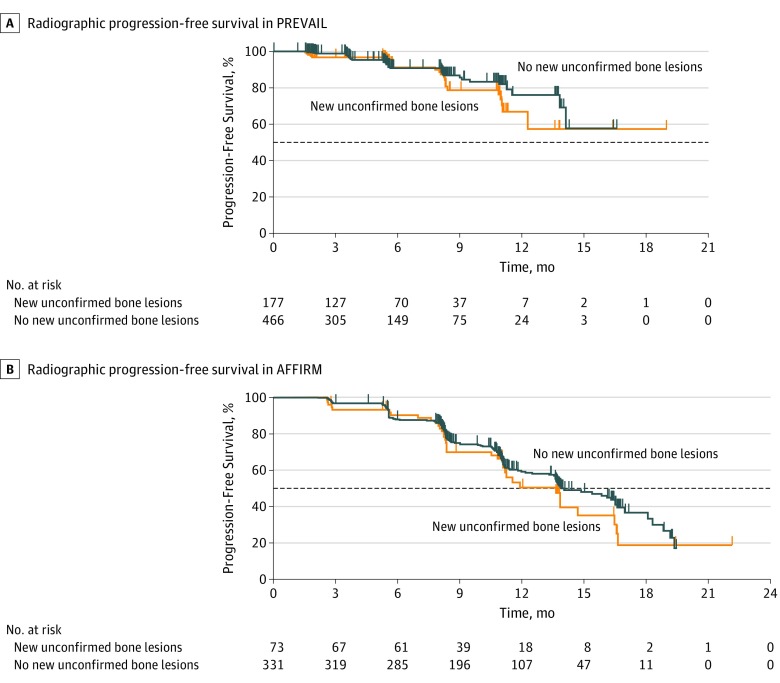
Figure 2. Radiographic Progression-Free Survival (rPFS) in PREVAIL and AFFIRM Among Men Treated With Enzalutamide Who Had a Decrease in Prostate-Specific Antigen Level or an Objective Soft-Tissue Response, With or Without New Unconfirmed Lesions Detected on Follow-up Bone Scans Over Time.
A, Median rPFS in PREVAIL among men with new unconfirmed bone lesions (n = 177), not reached (NR [95% CI, 12.3 months to NR]); and median rPFS in PREVAIL among men with no new unconfirmed bone lesions (n = 466), NR (95% CI, 14.1 months to NR); hazard ratio, 1.37 (95% CI, 0.81-2.30); P = .23. B, Median rPFS in AFFIRM among men with new unconfirmed bone lesions (n = 73), 13.6 months (95% CI, 11.1-16.5 months); and median rPFS in AFFIRM among men with no new unconfirmed bone lesions (n = 331), 13.9 months (95% CI, 13.6-16.5 months); hazard ratio, 1.21 (95% CI, 0.83-1.75); P = .32. Horizontal dashed lines indicate the median.