Key Points
Question
Are apolipoprotein E ε4 status and ball heading associated with verbal memory?
Findings
In this cross-sectional analysis of 352 amateur soccer players, those with greater exposure to ball heading in the prior 12 months and the apolipoprotein E ε4 allele demonstrated worse verbal memory than players with low exposure to ball heading.
Meaning
The findings from this study provide evidence to suggest that apolipoprotein E ε4 is a genetic risk factor for cognitive impairment associated with the high levels of long-term ball heading.
Abstract
Importance
Emerging evidence suggests that long-term exposure to ball heading in soccer, the most popular sport in the world, confers risk for adverse cognitive outcomes. However, the extent to which the apolipoprotein E ε4 (APOE ε4) allele, a common risk factor for neurodegeneration, and ball heading are associated with cognition in soccer players remains unknown.
Objective
To determine whether the APOE ε4 allele and 12-month ball heading exposure are associated with verbal memory in a cohort of adult amateur soccer players.
Design, Settings, and Participants
A total of 379 amateur soccer players were enrolled in the longitudinal Einstein Soccer Study from November 11, 2013, through January 23, 2018. Selection criteria included participation in soccer for more than 5 years and for more than 6 months per year. Of the 379 individuals enrolled in the study, 355 were genotyped. Three players were excluded for reporting extreme levels of heading. Generalized estimating equation linear regression models were employed to combine data across visits for a cross-sectional analysis of the data.
Exposures
At each study visit every 3 to 6 months, players completed the HeadCount 12-Month Questionnaire, a validated, computer-based questionnaire to estimate 12-month heading exposure that was categorized as low (quartiles 1 and 2), moderate (quartile 3), and high (quartile 4).
Main Outcome and Measures
Verbal memory was assessed at each study visit using the International Shopping List Delayed Recall task from CogState.
Results
A total of 352 soccer players (256 men and 96 women; median age, 23 years [interquartile range, 21-28 years]) across a total of 1204 visits were analyzed. High levels of heading were associated with worse verbal memory performance (β = −0.59; 95% CI, −0.93 to −0.25; P = .001). There was no main association of APOE ε4 with verbal memory (β = 0.09; 95% CI, −0.24 to 0.42; P = .58). However, there was a significant association of APOE ε4 and heading with performance on the ISRL task (χ2 = 7.22; P = .03 for overall interaction). In APOE ε4–positive players, poorer verbal memory associated with high vs low heading exposure was 4.1-fold greater (APOE ε4 negative, β = −0.36; 95% CI, −0.75 to 0.03; APOE ε4 positive, β = −1.49; 95% CI, −2.05 to −0.93), and poorer verbal memory associated with high vs moderate heading exposure was 8.5-fold greater (APOE ε4 negative, β = −0.13; 95% CI, −0.54 to 0.29; APOE ε4 positive, β = −1.11, 95% CI, −1.70 to −0.53) compared with that in APOE ε4–negative players.
Conclusions and Relevance
This study suggests that the APOE ε4 allele is a risk factor for worse memory performance associated with higher heading exposure in the prior year, which highlights that assessing genetic risks may ultimately play a role in promoting safer soccer play.
This cross-sectional study examines whether the apolipoprotein E ε4 allele and 12-month ball heading exposure are associated with verbal memory in a cohort of adult amateur soccer players.
Introduction
Soccer players, comprising an estimated 265 people million globally,1 are exposed to repetitive head impacts that do not result in clinical symptoms necessary for a diagnosis of concussion.2 Emerging evidence indicates that long-term exposure to subconcussive ball heading is associated with worse neuropsychological performance (NP).3,4,5,6,7
Apolipoprotein E (ApoE) is the major lipid transport protein in the central nervous system that facilitates cell membrane maintenance and repair, the formation of new synapses, and axonal and dendritic growth. The APOE gene (GenBank NCBI NG_007084.2) has 2 missense variants at amino acid residues 112 and 158 leading to 3 common haplotypes, which are typically referred to as APOE alleles ε2 (Cys and Cys), ε3 (Cys and Arg), and ε4 (Arg and Arg).8 More than 20 years ago, the APOE4 ε4 allele of the APOE gene was identified as a major genetic risk factor for Alzheimer disease.9 There is also evidence linking APOE ε4 to worse NP in boxers10 and US football players11; however, the role of APOE ε4 in modulating NP in association with subconcussive soccer heading has yet to be explored.
The goal of the present study is to examine the extent to which the presence of the APOE ε4 polymorphism and 12-month exposure to subconcussive heading are associated with NP in amateur soccer players. We specifically examined memory given prior evidence suggesting that the APOE ε4 allele is associated with worse memory in healthy aging adults.12,13 Furthermore, 2 independent samples previously demonstrated that verbal memory is the specific neuropsychological domain worsened by 12-month heading exposure.5,6 We hypothesized that soccer players who carry at least 1 copy of the APOE ε4 allele would demonstrate a stronger association between 12-month heading and worse verbal memory performance compared with peers without an APOE ε4 allele.
Methods
Participants
Population
A total of 379 amateur soccer players were enrolled in the longitudinal Einstein Soccer Study from November 11, 2013, through January 23, 2018. Details of recruitment and screening for the Einstein Soccer Study are described elsewhere.14 Players were eligible if they were aged 18 to 55 years, played soccer for more than 5 years, were currently playing soccer more than 6 months per year, and were fluent in English. Exclusion criteria included a self-reported diagnosis of schizophrenia, bipolar disorder, or a known neurologic disorder (eg, stroke or transient ischemic attack) or illicit drug use within 30 days based on history and results of a urine toxicology test so as to represent a population of healthy, adult amateur soccer players and mitigate the potential for bias on cognitive assessments. All study procedures were approved by the Albert Einstein College of Medicine Institutional Review Board. All participants provided written informed consent.
Study Procedures
A detailed description of the study procedures for the Einstein Soccer Study are described elsewhere.6,14 In brief, a research team member (P.S. or S.H.) contacted qualifying individuals, confirmed eligibility and willingness to participate in the full longitudinal study, and invited them to enroll. Refusals were defined as individuals who completed a screening form but could not be reached afterward, refused to participate, or withdrew from the study without completing their first study visit. Potential refusals were defined as individuals who did not consent to the screening form or who consented to the screening form but did not complete the form. At enrollment during the initial study visit, participants completed (1) written informed consent, (2) a web-based demographic questionnaire (eg, regarding sex, race/ethnicity, and years of education), (3) the HeadCount 12-Month Questionnaire (HeadCount-12m), (4) CogState, and (5) venipuncture to obtain blood samples for genotyping. Participants returned for follow-up visits every 3 to 6 months. Identical procedures, except consent and genotyping, were performed at each follow-up visit.
Assessments
HeadCount-12m
HeadCount-12m, a validated, computer-based questionnaire to estimate soccer heading, is described elsewhere in detail.5,6 Players completed the HeadCount-12m at each study visit and reflected on heading exposure in the prior year. In brief, participants are asked a series of questions pertaining to their soccer play during practice and competition in indoor and outdoor settings: (1) the number of months per year active in each setting, (2) the mean number of competitive soccer games per week, (3) the mean number of headers per game, (4) the mean number of practices per week, and (5) the mean number of headers per practice. The total number of headers in the past year was estimated by multiplying the mean number of headers in each setting by the number of sessions per week in each setting, converted to month, and then multiplying by the number of months of play per year. Subtotals in each setting were summed to obtain an estimate of total 12-month heading. The HeadCount-12m also asks participants to report the number of years that they have been playing soccer at a similar frequency and their lifetime concussion history. Participants were instructed to consider a concussion as any head injury for which they sought or were asked to seek medical attention.
Neuropsychological Performance
Verbal Memory: CogState (CogState Ltd), a valid and reliable computer-administered battery of cognitive function, was administered at every visit as part of a battery of tests assessing neuropsychological peformance.15 For the present analyses, we used the International Shopping List Delayed Recall (ISRL) task, a 12-item list learning task whereby participants are presented grocery shopping list items (eg, orange and chocolate) at the beginning of the testing session that they are instructed to recall 20 minutes later at the end of the testing session. The primary outcome measure for the ISRL is number of correct items (range, 0-12).
APOE Genotyping
At enrollment, 5 mL of whole blood was obtained via venipuncture. One of the APOE gene variants (rs7412) was genotyped using the Global Screening Array-24.v1.0 with a genotyping call rate of 98.5%. Only samples with a genotype call rate of more than 95% on the Global Screening Array chip were retained for further analysis. The other gene variant (rs429358) was genotyped using TaqMan at a genotyping call rate of 99.3%. The TaqMan assay was used for rs429358 because we identified a higher genotyping error rate for this variant on the Global Screening Array chip in our quality control step using samples with previously known APOE genotypes. Genotype data were analyzed with the Golden Helix SVS software (Golden Helix). The genotypes did not deviate from Hardy-Weinberg equilibrium.
Statistical Analysis
Baseline demographic characteristics were compared between heading groups using the Kruskal-Wallis or analysis of variance test for continuous variables and the χ2 or Fisher exact test for categorical variables. Generalized estimating equation models with an independent working correlation structure were fit to the data to examine the independent and interactive associations of 12-month heading and the APOE ε4 allele with the ISRL and to account for the within-participant correlation in repeated measures.16 This analysis approach permits the pooling of longitudinal data into a cross-sectional analysis. We used a dominant model given that only 1% of our sample were ε4 homozygotes. Owing to positive skew, 12-month heading was categorized into quartiles. The first and second quartiles were combined into a low-exposure group because a previous study has demonstrated that these groups do not differ with respect to NP,6 and the mean difference in ISRL scores between heading quartiles 1 and 2 was very close to zero (β = −0.008; 95% CI, −0.31 to 0.29; P = .96). The third and fourth quartiles were defined as moderate- and high-exposure group, respectively. In the event of a significant interaction, post hoc analyses stratified by APOE ε4 status were additionally conducted. Given published evidence that the APOE ε2 allele is neuroprotective,17 we conducted a sensitivity analysis by excluding individuals with the ε2 and ε4 genotype. All generalized estimating equation models were adjusted for age, sex, and educational level. Other potential confounders considered for inclusion in the models were race/ethnicity, past or present history of smoking, mean number of alcoholic drinks consumed in a week, number of years playing soccer at a similar frequency, age × heading interaction, and number of lifetime concussions (0, 1, or ≥2). Likewise, we fit an additional model stratified by sex and conducted a sensitivity analysis, including only white players to further adjust for the associations of these factors. The ISRL score in APOE ε4–negative players vs APOE ε4–positive players within each racial/ethnic category was tested. Baseline concussion was chosen as a covariate given that the mean (SD) numbers of concussions reported by participants at baseline and at 2 years are similar (baseline, 0.550 [0.09]; and 2 years, 0.546 [0.09]; P = .97). A backward stepwise selection approach was used to determine which of these confounders would be retained in the final models. All analyses were performed using Stata, version 15.0 (StataCorp LLC). A 2-sided P < .05 was considered statistically significant for all analyses.
Results
Demographic Characteristics
A total of 657 soccer players visited the study web portal through January 23, 2018; 119 explicitly refused to participate, and 159 did not provide data and were assumed to have refused. We successfully obtained blood samples from 355 of the remaining 379 players (94%). Three players were excluded from the present analysis because they reported more than 100 000 headers per year and were therefore defined as extreme outliers. Our final sample consisted of 352 soccer players, of whom 256 (73%) were men and 81 (23%) were APOE ε4 carriers (67 [19%] were ε3 and ε4 carriers, 10 [3%] were ε2 and ε4 carriers, and 4 [1%] were ε4 and ε4 carriers) (Table 1). The median age of players at baseline was 23 years (interquartile range [IQR], 21-28 years). Exposure groups defined by baseline levels of 12-month heading differed with respect to age, sex, educational level, alcohol use, and baseline CogState ISRL score. The median age was lowest for players in the fourth quartile of heading (21 years [IQR, 20-24 years]) compared with those in quartiles 1 and 2 (26 years [IQR, 22-33 years]) and quartile 3 (24 years [IQR, 21-31 years]). The mean (SD) number of years of education was lowest for players in quartile 4 (14.7 [2.3] years) compared with those in quartiles 1 and 2 (16.6 [2.6] years) and quartile 3 (15.7 [3.2] years). Compared with players in quartiles 1 and 2, players in quartile 3 and quartile 4 had lower mean (SD) CogState ISRL scores (first and second quartiles, 10.0 [1.7]; third quartile, 9.3 [1.8]; fourth quartile, 9.2 [1.9]). The percentage of men was lower in quartiles 1 and 2 (68 of 121 [56%]) compared with those in quartile 3 (82 of 101 [81%]) and quartile 4 (106 of 130 [82%]). The demographic characteristics were similar between APOE ε4–negative players and APOE ε4–positive players (Table 2).
Table 1. Baseline Demographics and Exposure Characteristics.
| Variable | Value | P Value | |||
|---|---|---|---|---|---|
| Total Sample (N = 352) | Heading Quartile | ||||
| 1 and 2 (n = 121) | 3 (n = 101) | 4 (n = 130) | |||
| No. of 12-mo headers, median (IQR) | 165 (70-296) | 638 (522-791) | 2346 (1538-3824) | ||
| Age, median (IQR), y | 23 (21-28) | 26 (22-33) | 24 (21-31) | 21 (20-24) | <.001 |
| Educational level, mean (SD), y | 15.6 (2.8) | 16.6 (2.6) | 15.7 (3.2) | 14.7 (2.3) | <.001 |
| CogState ISRL score, mean (SD) | 9.5 (1.8) | 10.0 (1.7) | 9.3. (1.8) | 9.2 (1.9) | <.001 |
| Years playing soccer at similar frequency, median (IQR)a | 11 (7-16) | 10 (5-16) | 12 (8-18) | 11 (7-15) | .26 |
| Male sex, No. (%) | 256 (73) | 68 (56) | 82 (81) | 106 (82) | <.001 |
| Race/ethnicity, No. (%) | |||||
| White | 235 (67) | 87 (72) | 73 (72) | 75 (58) | .17 |
| African American | 61 (17) | 18 (15) | 11 (11) | 32 (25) | |
| Asian | 20 (6) | 7 (6) | 6 (6) | 7 (5) | |
| Native Hawaiian or Pacific Islander | 4 (1) | 1 (1) | 1 (1) | 2 (2) | |
| Declined to answer | 32 (9) | 8 (7) | 10 (10) | 14 (11) | |
| Past or present smoker, No. (%) | 102 (29) | 39 (32) | 31 (31) | 32 (25) | .37 |
| Alcoholic drinks per wk, No. (%) | |||||
| 0 | 84 (24) | 19 (16) | 23 (23) | 42 (32) | .04 |
| 1-2 | 149 (42) | 52 (43) | 41 (41) | 56 (43) | |
| 3-7 | 93 (26) | 38 (31) | 31 (31) | 24 (19) | |
| 8-14 | 24 (7) | 11 (9) | 6 (6) | 7 (5) | |
| >14 | 2 (1) | 1 (1) | 0 | 1 (1) | |
| Lifetime concussion(s), No. (%)a | |||||
| 0 | 233 (66) | 78 (64) | 63 (62) | 82 (63) | .44 |
| 1 | 56 (16) | 23 (19) | 17 (17) | 16 (12) | |
| ≥2 | 67 (19) | 18 (15) | 20 (20) | 29 (21) | |
| APOE genotype, No. (%)b | |||||
| ε2/ε2 | 2 (1) | 1 (1) | 0 | 1 (1) | .91 |
| ε2/ε3 | 53 (15) | 21 (17) | 14 (14) | 18 (14) | |
| ε2/ε4 | 10 (3) | 4 (3) | 2 (2) | 4 (3) | |
| ε3/ε3 | 215 (61) | 69 (57) | 66 (65) | 80 (62) | |
| ε3/ε4 | 67 (19) | 25 (21) | 18 (18) | 24 (19) | |
| ε4/ε4 | 4 (1) | 1 (1) | 0 | 3 (2) | |
Abbreviations: APOE, apolipoprotein E; IQR, interquartile range; ISRL, International Shopping List Delayed Recall.
Data missing in 6 participants.
Variant (rs7412) failed in 1 participant (T-T homozygote).
Table 2. Baseline Characteristics in APOE ε4–Negative vs APOE ε4–Positive Players.
| Characteristic | Players | P Value | |
|---|---|---|---|
| APOE ε4 Negative (n = 271) | APOE ε4 Positive (n = 81) | ||
| Age, median (IQR), y | 23 (21-29) | 23 (21-27) | .64 |
| Educational level, mean (SD), y | 15.7 (2.7) | 15.6 (3.2) | .86 |
| Years playing soccer at similar frequency, median (IQR)a | 11 (6-16) | 1.5 (8-16) | .49 |
| CogState ISRL score, mean (SD) | 9.5 (1.8) | 9.5 (1.8) | .86 |
| Male sex, No. (%) | 198 (73) | 58 (72) | .80 |
| Race/ethnicity, No. (%) | |||
| White | 185 (68) | 50 (62) | .10 |
| African American | 39 (14) | 22 (27) | |
| Asian | 16 (6) | 4 (5) | |
| Native Hawaiian or Pacific Islander | 4 (1) | 0 | |
| Declined to answer | 27 (10) | 5 (6) | |
| Past or present smoker, No. (%) | 84 (31) | 18 (22) | .13 |
| Alcoholic drinks per wk, No. (%) | |||
| 0 | 62 (23) | 22 (27) | .51 |
| 1-2 | 116 (43) | 33 (41) | |
| 3-7 | 75 (28) | 18 (22) | |
| 8-14 | 17 (6) | 7 (9) | |
| >14 | 1 (0.4) | 1 (1) | |
| Lifetime concussion(s), No. (%)a | |||
| 0 | 166 (61) | 57 (70) | .20 |
| 1 | 48 (18) | 8 (10) | |
| ≥2 | 52 (19) | 15 (19) | |
Abbreviations: APOE, apolipoprotein E; IQR, interquartile range; ISRL, International Shopping List Delayed Recall.
Data missing in 6 participants.
Heading Activity
Baseline 12-month heading exposure was similar among APOE ε4–negative players (median, 661 [IQR, 296-1739]) and APOE ε4–positive players (median, 621 [IQR, 274-1738]; P = .98). The number of players who returned for follow-up visits were as follows: 40 players at 3 months, 243 players at 6 months, 52 players at 9 months, 194 players at 1 year, 29 players at 15 months, 156 players at 18 months, 6 players at 21 months, and 132 at 24 months.
Association of APOE Genotype and Heading With Verbal Memory
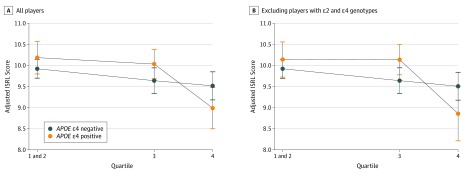
Consistent with a previous report,6 high levels of heading were associated with worse verbal memory performance (β = −0.59; 95% CI, −0.93 to −0.25; P = .001) (Table 3). There was no main association of APOE ε4 with verbal memory (β = 0.09; 95% CI, −0.24 to 0.42; P = .58). However, there was a significant association of APOE ε4 and heading with performance on the ISRL task (χ2 = 7.22; P = .03 for overall interaction). In analyses stratified by APOE ε4 status, the APOE ε4–positive players demonstrated a 4.1-fold greater deficit in verbal memory associated with high vs low heading exposure (APOE ε4 negative, β = −0.36; 95% CI, −0.75 to 0.03; APOE ε4 positive, β = −1.49; 95% CI, −2.05 to −0.93) and an 8.5-fold greater deficit in verbal memory associated with high vs moderate heading exposure (APOE ε4 negative, β = −0.13; 95% CI, −0.54 to 0.29; APOE ε4 positive, β = −1.11; 95% CI, −1.70 to −0.53) compared with APOE ε4–negative players (Table 3; Figure, A). To interpret the results on the absolute scale, the decrease in adjusted mean ISRL scores between the high and low heading exposure groups was 1.13 greater (1.49 − 0.36 = 1.13) in APOE ε4 carriers compared with noncarriers, and between the high- and moderate-exposure groups, it was 0.98 greater (1.11 − 0.13 = 0.98) in APOE ε4 carriers compared with noncarriers. In the sensitivity analysis excluding players with the ε2 and ε4 alleles, the association with APOE ε4 was even more pronounced; the APOE ε4–positive players demonstrated a 4.9-fold greater deficit in verbal memory associated with high vs low heading exposure (APOE ε4 positive, β = −1.75; 95% CI, –2.43 to –1.08; APOE ε4 negative, β = −0.36; 95% CI, −0.75 to 0.03) and a 10.9-fold greater deficit in verbal memory associated with high vs moderate heading exposure (APOE ε4 positive, β = −1.42; 95% CI, −2.16 to −0.69; APOE ε4 negative, β = −0.13; 95% CI, −0.54 to 0.29) (Table 4; Figure, B).
Table 3. Mean Difference in Verbal Memorya.
| Model | Main Effects Modelb | APOE ε4 Stratified Analysesc | ||||
|---|---|---|---|---|---|---|
| APOE ε4–Negative Playersb | APOE ε4–Positive Playersb | |||||
| β (95% CI)d | P Value | β (95% CI)d | P Value | β (95% CI)d | P Value | |
| International Shopping List Delayed Recalle | ||||||
| Model 1 (low heading exposure as reference group) | ||||||
| Heading exposure | ||||||
| Low | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Moderate | −0.25 (−0.54 to 0.04) | .09 | −0.23 (−0.59 to 0.12) | .19 | −0.37 (−0.82 to 0.08) | .10 |
| High | −0.59 (−0.93 to −0.25) | .001 | −0.36 (−0.75 to 0.03) | .07 | −1.49 (−2.05 to −0.93) | <.001 |
| APOE ε4 | 0.09 (−0.24 to 0.42) | .58 | NA | NA | NA | NA |
| Model 2 (moderate heading exposure as reference group) | ||||||
| Heading exposure | ||||||
| Low | 0.25 (−0.04 to 0.54) | .09 | 0.23 (−0.12 to 0.59) | .19 | 0.37 (−0.08 to 0.82) | .10 |
| Moderate | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| High | −0.34 (−0.69 to 0.01) | .06 | −0.13 (−0.54 to 0.29) | .55 | −1.11 (−1.70 to −0.53) | <.001 |
| APOE ε4 | 0.09 (−0.24 to 0.42) | .58 | NA | NA | NA | NA |
Abbreviations: APOE, apolipoprotein E; ISRL, International Shopping List Delayed Recall; NA, not applicable.
There were 352 players and 1204 observations.
Adjusted for sex, age, years of education, race/ethnicity, smoking history, and alcohol use.
P = .03 for APOE ε4 × heading interaction from the generalized estimating equation model.
β Values refer to the difference in ISRL score compared with the reference group.
Higher score indicates better performance.
Figure. Association Between 12-Month Heading and Verbal Memory Based on APOE ε4 Status.
A, Model shown in all players (N = 352) (P = .03 for overall interaction). B, Model excluding players with ε2 and ε4 genotypes (n = 342) (P = .03 for overall interaction). All models were adjusted for sex, age, years of education, race/ethnicity, smoking history, and alcohol use. ISRL indicates International Shopping List Delayed Recall.
Table 4. Estimated Mean Change in Verbal Memory Excluding Players With the APOE ε2 and ε4 Allelesa.
| Model | Main Effects Modelb | APOE ε4 Stratified Analysesc | ||||
|---|---|---|---|---|---|---|
| APOE ε4–Negative Playersb | APOE ε4–Positive Playersb | |||||
| β (95% CI)d | P Value | β (95% CI)d | P Value | β (95% CI)d | P Value | |
| International Shopping List Delayed Recalle | ||||||
| Model 1 (low heading exposure as reference group) | ||||||
| Heading exposure | ||||||
| Low | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Moderate | −0.22 (−0.52 to 0.08) | .15 | −0.23 (−0.59 to 0.12) | .19 | −0.33 (−0.76 to 0.10) | .13 |
| High | −0.57 (−0.93 to −0.21) | .002 | −0.36 (−0.75 to 0.03) | .07 | −1.75 (−2.43 to −1.08) | <.001 |
| APOE ε4 | 0.08 (−0.28 to 0.44) | .65 | NA | NA | NA | NA |
| Model 2 (moderate heading exposure as reference group) | ||||||
| Heading exposure | ||||||
| Low | 0.22 (−0.08 to 0.52) | .15 | 0.23 (−0.12 to 0.59) | .19 | 0.33 (−0.10 to 0.76) | .13 |
| Moderate | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| High | −0.35 (−0.72 to 0.02) | .07 | −0.13 (−0.54 to 0.29) | .55 | −1.42 (−2.16 to −0.69) | <.001 |
| APOE ε4 | NA | NA | NA | NA | NA | NA |
Abbreviations: APOE, apolipoprotein E; ISRL, International Shopping List Delayed Recall; NA, not applicable.
There were 342 players and 1151 observations.
Adjusted for sex, age, years of education, race/ethnicity, smoking history, and alcohol use.
P = .03 for APOE ε4 × heading interaction from the generalized estimating equation model.
β Values refer to the difference in ISRL score compared with the reference group.
Higher score indicates better performance.
In the analyses stratified by sex, no significant associations of heading with verbal memory were seen for women, likely owing to limited power (eTable 1 in the Supplement). The interactive model including only white players was not significant (χ2 = 4.97; P = .08); however, the trends were similar to those in the full model (eTable 2 in the Supplement). There were no significant memory differences among APOE ε4–negative players vs APOE ε4–positive players within each racial/ethnic category (eTable 3 in the Supplement).
Discussion
We directly examined the role of the APOE genotype in the association of repetitive, subconcussive head impacts in soccer with NP using a quantifiable measure of heading exposure. We found that APOE ε4 and 12-month heading exposure are significantly associated with verbal memory performance. Compared with APOE ε4–negative players, APOE ε4–positive players demonstrated worse verbal memory associated with 12-month heading. The magnitude of the association of APOE ε4 and higher levels of heading with memory performance was larger when we excluded players with the ε2 and ε4 alleles from the analysis, for whom the presence of the neuroprotective APOE ε2 allele may have mitigated the harm associated with the APOE ε4 allele.17
Our findings provide preliminary insight into an interaction between genetic and environmental factors associated with the risk for emerging subclinical cognitive impairment in soccer heading. These findings address an important knowledge gap because only limited research has explored the role of the APOE ε4 allele in NP from repetitive head impacts. Two studies, 1 in retired boxers10 and 1 in US football players,11 have shown worse cognitive outcomes associated with long-term exposure to repetitive head impacts in APOE ε4 carriers. These studies, however, did not explicitly quantify or estimate the amount of repetitive head impacts and did not attempt to disentangle the independent associations of injury severity (eg, subconcussive injury vs mild traumatic brain injury vs moderate or severe traumatic brain injury) with NP. Our findings provide the first indication, to our knowledge, that the APOE ε4 allele may be associated with adverse cognitive sequelae of subconcussive repetitive head impacts independent of prior concussion.
Prior research on repetitive head injuries in sports suggests that impairment follows a threshold pattern.2,18 Furthermore, previous reports in 2 independent populations suggest a nonlinear association between long-term heading and memory impairment wherein adverse cognitive outcomes emerge at high levels of exposure.5,6 In the present study, we provide evidence that APOE ε4–positive players are most vulnerable to this threshold effect. As evidenced in the Figure, at low levels of exposure, APOE ε4–positive players exhibit a nonsignificant trend toward better memory performance, whereas at high levels of heading, APOE ε4–positive players demonstrate a significant deficit in memory performance. Perhaps this pattern is due to the cumulative nature of APOE-dependent biological processes in the brain.19
The neurobiological mechanisms underlying the role of APOE ε4–specific response to traumatic brain injury outcomes have yet to be established. Responses may be associated with the ApoE4 isoform’s impaired function of lipid delivery that affects neurite outgrowth20 and synapse formation as well as specific neurotoxic effects in response to neuronal injuries.21 Furthermore, experimental studies suggest that APOE ε4–positive individuals are more susceptible to enhanced deposition and/or reduced clearance of amyloid, greater oxidative stress, deposition of hyperphosphorylated tau, impaired blood-brain barrier repair, and an augmented neuroinflammatory response.22,23,24,25,26 Future preclinical studies of repeated subconcussive injury in APOE transgenic mice are necessary to elucidate the specific pathologic mechanism(s) subserving the isoform-specific pattern of memory impairment demonstrated by our preliminary findings.
The effect size of our interaction is relatively small. However, similar to the widely cited model of disease evolution in Alzheimer disease,27 our findings may be evidence of early subclinical effects, which could accumulate in APOE ε4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction. Potential mechanisms could include the accumulation of injury pathologic characteristics that ultimately surpass a threshold to confer overt dysfunction or trigger a later-onset neurodegenerative process by earlier subclinical injury pathologic findings. In the former case, the subclinical injury effects would directly produce later overt clinical dysfunction. In the latter case, early subclinical effects could denote an inciting event that presages later onset of neurodegenerative disease in susceptible (APOE ε4–positive) players.
Strengths and Limitations
To our knowledge, this is the first study to examine the role of APOE ε4 in outcomes from soccer heading; however, several limitations must be considered in the interpretation of our findings. Our population consisted of adult, amateur soccer players in the United States and thus cannot be generalized to other demographic groups. However, our cohort has been noted to reflect the general composition of amateur soccer players.28 Furthermore, we used a structured self-report instrument for our estimation of 12-month heading exposure that may be subject to recall bias and does not capture biomechanical features of individual head impacts. This limitation is mitigated by the fact that the measure is well validated and has been demonstrated to predict heading outcomes.29 There were missing data in 6% of our sample for whom we were not able to obtain a blood sample for genotyping. Missing data may have biased our results. However, the mean verbal memory score in the missing sample (9.5) was identical to that of the participants included in our analysis. Moreover, most participants (54%) who were missing genotype data were in the fourth quartile of heading, which indicates that our results persisted despite potential bias toward the null. This analysis was cross-sectional, and therefore we cannot explicitly confirm causation. This study was not powered to detect a race/ethnicity × heading × gene interaction; however, race/ethnicity may play an important role in the interpretation of these findings. Finally, given our sample size, we used a dominant model. Larger future studies are necessary to examine the additive association of the APOE ε4 allele with outcomes and perhaps reveal a more robust effect size.
Conclusions
This study has addressed the growing movement toward identifying genetic factors associated with outcomes from head trauma.22 We provide preliminary evidence that carriers of the APOE ε4 allele are at greater risk of memory impairment associated with high levels of long-term soccer heading. These results suggest that recommendations for safe levels of soccer heading, such as advising APOE ε4–positive players to avoid or limit their exposure to repetitive head impacts, could be leveraged to design public health interventions that protect players from harm. Larger studies and longitudinal studies are necessary to characterize maximum levels of heading that can be considered safe in distinct subgroups of individuals defined by genetic risk.
eTable 1. Estimated Mean Change in Verbal Memory Stratified by Sex
eTable 2. Estimated Mean Change in Verbal Memory in Only White Players
eTable 3. Difference in Verbal Memory Score in APOE-ε4 (-) vs APOE-ε4 (+) by Racial Category
References
- 1.Kunz M. 265 Million playing football. FIFA Magazine July 2007:10-15. https://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Accessed December 9, 2019.
- 2.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg. 2013;119(5):1235-1245. doi: 10.3171/2013.7.JNS121822 [DOI] [PubMed] [Google Scholar]
- 3.Matser JT, Kessels AG, Lezak MD, Troost J. A dose-response relation of headers and concussions with cognitive impairment in professional soccer players. J Clin Exp Neuropsychol. 2001;23(6):770-774. doi: 10.1076/jcen.23.6.770.1029 [DOI] [PubMed] [Google Scholar]
- 4.Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18(4):397-417. doi: 10.1093/arclin/18.4.397 [DOI] [PubMed] [Google Scholar]
- 5.Lipton ML, Kim N, Zimmerman ME, et al. . Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268(3):850-857. doi: 10.1148/radiol.13130545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitch CF, Zimmerman ME, Lubin N, et al. . Recent and long-term soccer heading exposure is differentially associated with neuropsychological function in amateur players. J Int Neuropsychol Soc. 2018;24(2):147-155. doi: 10.1017/S1355617717000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart WF, Kim N, Ifrah C, et al. . Heading frequency is more strongly related to cognitive performance than unintentional head impacts in amateur soccer players. Front Neurol. 2018;9:240. doi: 10.3389/fneur.2018.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241-252. doi: 10.1016/S1474-4422(10)70325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, et al. . Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921-923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 10.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E ε4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278(2):136-140. doi: 10.1001/jama.1997.03550020068040 [DOI] [PubMed] [Google Scholar]
- 11.Kutner KC, Erlanger DM, Tsai J, Jordan B, Relkin NR. Lower cognitive performance of older football players possessing apolipoprotein E ε4. Neurosurgery. 2000;47(3):651-657. doi: 10.1097/00006123-200009000-00026 [DOI] [PubMed] [Google Scholar]
- 12.Caselli RJ, Dueck AC, Osborne D, et al. . Longitudinal modeling of age-related memory decline and the APOE ε4 effect. N Engl J Med. 2009;361(3):255-263. doi: 10.1056/NEJMoa0809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albrecht MA, Szoeke C, Maruff P, et al. ; AIBL Research Group . Longitudinal cognitive decline in the AIBL cohort: the role of APOE ε4 status. Neuropsychologia. 2015;75:411-419. doi: 10.1016/j.neuropsychologia.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Kim N, Ifrah CS, et al. . Symptoms from repeated intentional and unintentional head impact in soccer players. Neurology. 2017;88(9):901-908. doi: 10.1212/WNL.0000000000003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruff P, Thomas E, Cysique L, et al. . Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165-178. doi: 10.1093/arclin/acp010 [DOI] [PubMed] [Google Scholar]
- 16.Hardin JW. Generalized Estimating Equations. Hoboken, NJ: Wiley Online Library; 2005. [Google Scholar]
- 17.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neurosci Biobehav Rev. 2013;37(10, pt 2):2878-2886. doi: 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 18.Montenigro PH, Alosco ML, Martin BM, et al. . Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma. 2017;34(2):328-340. doi: 10.1089/neu.2016.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kok E, Haikonen S, Luoto T, et al. . Apolipoprotein E–dependent accumulation of Alzheimer disease–related lesions begins in middle age. Ann Neurol. 2009;65(6):650-657. doi: 10.1002/ana.21696 [DOI] [PubMed] [Google Scholar]
- 20.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264(5160):850-852. doi: 10.1126/science.8171342 [DOI] [PubMed] [Google Scholar]
- 21.Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76(5):871-885. doi: 10.1016/j.neuron.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem Res. 2007;32(4-5):905-915. doi: 10.1007/s11064-006-9251-3 [DOI] [PubMed] [Google Scholar]
- 23.Cao J, Gaamouch FE, Meabon JS, et al. . ApoE4-associated phospholipid dysregulation contributes to development of tau hyper-phosphorylation after traumatic brain injury. Sci Rep. 2017;7(1):11372. doi: 10.1038/s41598-017-11654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Main BS, Villapol S, Sloley SS, et al. . Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener. 2018;13(1):17. doi: 10.1186/s13024-018-0249-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng Z, Guo Z, Zhong J, et al. . ApoE influences the blood-brain barrier through the NF-κB/MMP-9 pathway after traumatic brain injury. Sci Rep. 2017;7(1):6649. doi: 10.1038/s41598-017-06932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keene CD, Cudaback E, Li X, Montine KS, Montine TJ. Apolipoprotein E isoforms and regulation of the innate immune response in brain of patients with Alzheimer’s disease. Curr Opin Neurobiol. 2011;21(6):920-928. doi: 10.1016/j.conb.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen B, Karceski S. Soccer and head injuries: what is the risk? Neurology. 2017;88(9):e74-e77. doi: 10.1212/WNL.0000000000003669 [DOI] [PubMed] [Google Scholar]
- 29.Lipton ML, Ifrah C, Stewart WF, et al. . Validation of HeadCount-2w for estimation of two-week heading: comparison to daily reporting in adult amateur player. J Sci Med Sport. 2018;21(4):363-367. doi: 10.1016/j.jsams.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Estimated Mean Change in Verbal Memory Stratified by Sex
eTable 2. Estimated Mean Change in Verbal Memory in Only White Players
eTable 3. Difference in Verbal Memory Score in APOE-ε4 (-) vs APOE-ε4 (+) by Racial Category