Abstract
Background
Dementia is a common mental health problem affecting 5% of those over 65. Various pathological processes are linked to memory impairment in dementia, particularly those affecting the cholinergic neurotransmitter system. Acetyl‐l‐carnitine (ALC) is derived from carnitine and is described as having several properties which may be beneficial in dementia. These include activity at cholinergic neurons, membrane stabilization and enhancing mitochondrial function. Work on the effects of ALC has been ongoing since the 1980s yet the mechanism of efficacy of ALC in cognitive decline remains unclear. Early studies suggested a beneficial effect of ALC on cognition and behaviour in aging subjects. However, later, larger studies have not supported these findings. Some of the difficulties lie in the differences in methodology and assessment tools used in the early and later studies. They are therefore difficult to compare. ALC is not currently in routine clinical use.
Objectives
To establish whether Acetyl‐l‐carnitine is clinically effective in the treatment of people with dementia.
Search methods
The trials were identified from a search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group, The Cochrane Library, EMBASE, MEDLINE, CINAHL, PsycINFO and LILACS on 8 November using the terms acetyl‐l‐carnitine, l‐carnitine acetyl ester, acetylcarnitine, ALC. The search in November 2007 revealed no new studies.
Selection criteria
All double‐blind, randomized, trials involving people with dementia in which treatment with ALC was compared with a placebo group
Data collection and analysis
Data were extracted by a reviewer (SH) and entered into RevMan 4.2 software. Where possible intention‐to‐treat data were used, but most of the analyses were of completers (people who completed the study).
Main results
There are sixteen included trials, all of which included participants with mild‐moderate dementia or cognitive decline. All trials assessed the cognitive effects of ALC and in addition most considered severity of dementia, functional ability and clinical global impression. When considering clinical global impression (CGI‐I) as a dichotomous variable (numbers improved versus numbers unchanged or worse) there were statistically significant treatment effects in favour of ALC at 12 and 24 weeks, (Peto odds ratio (OR) 1.90, 95% Confidence Interval (CI) 1.31 to 2.76) and (OR 2.33, 95% CI 1.31 to 4.14) but not at 52 weeks (OR 0.91, 95% CI 0.58 to 1.43). There was also a statistically significant treatment effect on MMSE at 24 weeks (Weighted Mean Difference (WMD) 0.69, 95% CI 0.09 to 1.29, P = 0.02), but not at 12 or 52 weeks. There was no evidence of benefit of ALC in the areas of severity of dementia, functional ability or Clinical Global Impression as a continuous measure. Various adverse events were reported, but from the meta‐analyses there were no statistically significant differences between treated and placebo groups.
Authors' conclusions
There is evidence for benefit of ALC on clinical global impression as a categorical measure and on MMSE at 24 weeks, but there is no evidence using objective assessments in any other area of outcome. Given the large number of comparisons made, the statistically significant results may be due to chance. At present there is no evidence to recommend its routine use in clinical practice. Many of the trials used rather vague descriptions of dementia and trials using more strictly defined groups may be informative. Individual patient data may add to the findings, as would trials including other outcomes (e.g. mood and caregiver quality of life). However, the evidence does not suggest that ALC is likely to prove an important therapeutic agent. More work on the pharmacokinetics of ALC in humans is also required.
Plain language summary
No evidence of benefit of Acetyl‐l‐carnitine for dementia
Acetyl‐l‐carnitine (ALC) is derived from carnitine and is described as having several properties which may be beneficial in dementia. Early studies suggested a beneficial effect of ALC on cognition and behaviour in aging subjects. However, later, larger studies have not supported these findings. The early and later studies differ widely in methodology and assessment tools used, and are therefore difficult to compare. There is no evidence of benefit of ALC in the areas of cognition, severity of dementia, functional ability or Clinical Global Impression as a continuous measure. An apparent beneficial effect on Clinical Global Impression assessed as a dichotomous variable may be due to chance. There was also a significant treatment effect on the Mini Mental State Examination (MMSE) at 24 weeks, but this result must be interpreted with caution in the context of significant heterogeneity in these trials. ALC is not currently in routine clinical use.
Background
Dementia is a common and serious mental health problem affecting 5% of individuals over the age of 65 and 20% of those over the age of 80 (Jorm 1987). The clinical features of dementia include an acquired global impairment of intellect, memory and changes in personality. Although characteristic neuropathological findings may differ between types of dementia (Alzheimer's disease, Vascular dementia, Lewy body dementia and others), the memory impairment observed in most cases is suspected of being linked to the loss of cholinergic neurons. Indeed, studies have demonstrated both loss of cholinergic neurons and lower levels of the synthetic enzyme choline acetyl transferase in the brains of people affected by dementia (Davies 1976; Whitehouse 1982). Another clear finding is of widespread cell death and synaptic membrane damage, which may be mediated by abnormal energy processing and the production of free radicals.
Carnitine (and its racemic form l‐carnitine) is an endogenous substance, which participates in the synthesis of natural chemical products of the cell by facilitating transacetylation. It also functions as a shuttle between the cytoplasm and mitochondria for long chain fatty acids. Carnitine is present in cells and tissues as free carnitine and as acyl‐derivatives, of which ALC is one. The enzymes involved in the synthesis of ALC are the carnitine acyl‐transferases, and in particular carnitine acetyl‐transferase (Pettegrew 2000). ALC is described (in its official registration document) as a drug agonist of mitochondrial function, a neuronal growth factor and with an antioxidant effect on CNS neurons. The effect of ALC on cholinergic neurotransmission is unclear. An intrinsic cholinergic activity of ALC has not been reliably proven: in a paper on the analgesic effects of ALC, Bartolini states that "a postsynaptic mechanism can be ruled out" (Ghelardini 2002). A presynaptic mechanism is proposed, as ALC induces an increase in acetylcholine release, as measured by microdialysis in the caudate‐putamen and hippocampus (Imperato 1989). In addition, it may promote membrane stabilization, via its ability to increase adenosine levels.
ALC has multiple neurobiological effects which may possibly be beneficial in degenerative brain disease. These are helpfully reviewed in Pettegrew 2000. Animal studies have indicated that ALC normalized alterations in cerebral membrane and energy metabolism during recovery from ischaemia (Aureli 1991; Rosenthal 1992). It has also been reported that ALC prolonged survival of cultured rat brain cells after exposure to neurotoxic stimuli (Forloni 1994) and improved cognitive functioning in rats (Girardi 1992). Oxidative mitochondrial decay contributes to aging and cognitive decline. Some of this mitochondrial decay can be reversed in old rats by feeding them with ALC (Ames 2004).
In human studies, ALC improved brain measures of membrane phospholipid and high‐energy phosphate metabolism which corresponded with a delay in subjects' cognitive decline (Pettegrew 1995). Although currently it is not in routine clinical use, ALC has been found to be safe in dementia patients with few reported adverse effects (Sano 1992; Spagnoli 1991a). It has also been used to treat older patients with depression (Bella 1990; Fulgente 1990; Garzya 1990) and fatigue symptoms (Tomassini 2004). Clinical studies have been performed in association with the Italian pharmaceutical company producing ALC as ALCAR (Sigma‐Tau) since the 1980s. These studies were on the cognitive and behavioural effects of ALC in ageing subjects. Encouraging findings in these small, uncontrolled and often unpublished studies led to a series of small controlled studies, which suggested a possible beneficial role for ALC in slowing down cognitive decline in subjects with cognitive impairment. However, a more recent and larger study was less conclusive in its findings (Thal 1996). A recent review and meta‐analysis found a significant advantage for ALC compared to placebo (Montgomery 2003). Therefore, the purpose of this updated review is to consider whether ALC is effective for people with dementia.
Objectives
To establish whether acetyl‐l‐carnitine is clinically effective in the treatment of people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
Only double‐blind randomized controlled trials assessing the effectiveness of ALC in dementia patients were included. Trials were only included if treatment with ALC was compared with a placebo control group. Included trials had to show evidence of randomization of allocation to treatment or control group, and also concealment of this allocation.
Types of participants
Individuals with a clinical diagnosis of any type of dementia and of any severity. Later trials were expected to make a diagnosis of dementia according to internationally accepted guidelines such as DSM (DSM III‐R; DSM‐IV) or NINCDS‐ADRDA (McKhann 1984). However, at the time earlier trials were carried out these guidelines were not in common use. In these cases careful assessment was made of the diagnostic criteria used, and trials were included if the criteria seemed likely to include people with dementia.
Types of interventions
1. ALC at any dose 2. Placebo
Types of outcome measures
Clinical global impression of change
Global severity of dementia
Cognition (as measured by psychometric tests)
Behaviour
Mood
Activities of daily living
Institutionalization
Acceptability of treatment (as measured by withdrawal from trial)
Safety (as measured by incidence of adverse effects)
Mortality
Search methods for identification of studies
See Cochrane Dementia and Cognitive Improvement Group methods used in reviews.
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) was searched on 8 November 2007 for all years up to December 2005. This register contains records from the following major healthcare databases The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing trial databases and other grey literature sources. The following search terms were used: acetyl‐l‐carnitine OR L‐carnitine acetyl ester OR acetylcarnitine OR ALC.
The Cochrane Library, MEDLINE, EMBASE, PsycINFO and CINAHL were searched separately on 8 November 2007 for records added to these databases after December 2005 to November 2007. The search terms used to identify relevant controlled trials on dementia, Alzheimer's disease and mild cognitive impairment for the Group's Specialized Register can be found in the Group's module on The Cochrane Library. These search terms were combined with the following search terms and adapted for each database, where appropriate: acetyl‐l‐carnitine OR L‐carnitine acetyl ester OR acetylcarnitine OR ALC.
The search in November 2007 revealed no new studies.
On 8 November 2007, the Specialized Register consisted of records from the following databases:
Healthcare databases
CENTRAL: (The Cochrane Library 2006, Issue 1);
MEDLINE (1966 to 2006/07, week 5);
EMBASE (1980 to 2006/07);
PsycINFO (1887 to 2006/08, week 1);
CINAHL (1982 to 2006/06);
SIGLE (Grey Literature in Europe) (1980 to 2005/03);
LILACS: Latin American and Caribbean Health Science Literature (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) (last searched 29 August 2006).
Conference proceedings
ISTP (http://portal.isiknowledge.com/portal.cgi) (Index to Scientific and Technical Proceedings) (to 29 August 2006);
INSIDE (BL database of Conference Proceedings and Journals) (to June 2000);.
Theses
Index to Theses (formerly ASLIB) (http://www.theses.com/) (UK and Ireland theses) (1716 to 11 August 2006);
Australian Digital Theses Program (http://adt.caul.edu.au/): (last update 24 March 2006);
Canadian Theses and Dissertations (http://www.collectionscanada.ca/thesescanada/index‐e.html): 1989 to 28 August 2006);
DATAD ‐ Database of African Theses and Dissertations (http://www.aau.org/datad/backgrd.htm);
Dissertation Abstract Online (USA) (http://wwwlib.umi.com/dissertations/gateway) (1861 to 28 August 2006).
Ongoing trials
UK
National Research Register (http://www.update‐software.com/projects/nrr/) (last searched issue 3/2006);
ReFeR (http://www.refer.nhs.uk/ViewWebPage.asp?Page=Home) (last searched 30 August 2006);
Current Controlled trials: Meta Register of Controlled trials (mRCT) (http://www.controlled‐trials.com/) (last searched 30 August 2006) :
ISRCTN Register ‐ trials registered with a unique identifier
Action medical research
Kings College London
Laxdale Ltd
Medical Research Council (UK)
NHS Trusts Clinical Trials Register
National Health Service Research and Development Health Technology Assessment Programme (HTA)
National Health Service Research and Development Programme 'Time‐Limited' National Programmes
National Health Service Research and Development Regional Programmes
The Wellcome Trust
Stroke Trials Registry (http://www.strokecenter.org/trials/index.aspx) (last searched 31 August 2006);
Netherlands
Nederlands Trial Register (http://www.trialregister.nl/trialreg/index.asp) (last searched 31 August 2006);
USA/International
ClinicalTrials.gov (http://www.ClinicalTrials.gov) (last searched 31 August 2006) (contains all records from http://clinicalstudies.info.nih.gov/);
IPFMA Clinical trials Register: www.ifpma.org/clinicaltrials.html. The Ongoing Trials database within this Register searches http://www.controlled‐trials.com/isrctn, http://www.ClinicalTrials.gov and http://www.centerwatch.com/. The ISRCTN register and Clinicaltrials.gov are searched separately. Centerwatch is very difficult to search for our purposes and no update searches have been done since 2003.
The IFPMA Trial Results databases searches a wide variety of sources among which are:
http://www.astrazenecaclinicaltrials.com (seroquel, statins)
http://www.centerwatch.com
http://www.clinicalstudyresults.org
http://clinicaltrials.gov
http://www.controlled‐trials.com
http://ctr.gsk.co.uk
http://www.lillytrials.com (zyprexa)
http://www.roche‐trials.com (anti‐abeta antibody)
http://www.organon.com
http://www.novartisclinicaltrials.com (rivastigmine)
http://www.bayerhealthcare.com
http://trials.boehringer‐ingelheim.com
http://www.cmrinteract.com
http://www.esteve.es
http://www.clinicaltrials.jp
This part of the IPFMA database is searched and was last updated on 4 September 2006;
Lundbeck Clinical Trial Registry (http://www.lundbecktrials.com) (last searched 15 August 2006);
Forest Clinical trial Registry (http://www.forestclinicaltrials.com/) (last searched 15 August 2006).
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module on The Cochrane Library.
Data collection and analysis
Selection of studies
A single reviewer (SH) examined all the references retrieved by the electronic search and other methods outlined above. Many studies were clearly not relevant and subsequently discarded on the basis of their abstracts. Studies which were relevant, or of uncertain relevance, were retrieved. These studies were examined by both reviewers (SH, NT) independently and considered for inclusion using predetermined criteria. The reviewers then discussed any discrepancies and agreed on a final set of studies to be included.
Quality assessment
The quality of the methodology of each selected trial was examined with reference to the Cochrane Collaboration guidelines. Important aspects of the methodology considered were blinding, placebo control, reporting of dropouts and randomization. In terms of randomization, if the methods were clearly described and concealment was felt to be adequate they were termed 'A'. If concealment of allocation appeared to be inadequate, or was mentioned but not described these were classed as 'B'. 'C' studies were randomized but treatment was felt to be inadequately concealed.
Data collection
Data for the meta‐analyses were collected from each study using a standard form and entered onto RevMan 4.2 software. For the intention‐to‐treat analyses, data were sought for each outcome measure on every patient randomized, irrespective of compliance. Where the intention‐to‐treat data were not reported, data were sought on every patient who completed the study for the analyses of completers. In most cases the data were of completers.
Data were extracted from the published reports. The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at each time point were extracted. The baseline assessment is defined as the latest available assessment prior to randomization, but no longer than two months before.
For binary data the numbers in each treatment group and the numbers experiencing the outcome of interest were sought. For the global impression of change, the endpoint itself is of clinical relevance as all patients were by definition at the same baseline score.
Data analysis
The outcomes measured in clinical trials of dementia and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the trials have a reasonably large number of categories (more than 10) the data were treated as continuous outcomes arising from a normal distribution.
Summary statistics (n, mean and standard deviation) were required for each rating scale at each assessment time for each treatment group in each trial for change from baseline. When changes from baseline results were not reported, the required summary statistics were calculated from the baseline and assessment time treatment group means and standard deviations. In this case a zero correlation between the measurements at baseline and assessment time was assumed. This method overestimates the standard deviation of the change from baseline, but this conservative approach is considered to be preferable in a meta‐analysis.
The meta‐analysis requires the combination of data from the trials that use the same rating scale to assess an outcome. Selected studies evaluated a variety of outcome measures, using a range of assessments and scales. This meant that the data from all the studies could not be readily analysed as a group according to outcome measure. Despite this the different outcomes could be sensibly grouped under several broad headings. These broad outcome categories were: cognition, functional ability (ability to perform activities of daily living), severity of dementia and clinical global impression of change. The measure of the treatment difference for any outcome was the weighted mean difference (WMD) when the pooled trials use the same rating scale or test, and the standardised mean difference (SMD), which is the absolute mean difference divided by the standard deviation, when they used different rating scales or tests.
The duration of the trials varied considerably. Where the range was considered too great to combine all trials into one meta‐analysis it was divided into smaller time periods and a separate meta‐analysis conducted for each period.
For binary outcomes ‐ such as dead or alive, clinical improvement or no clinical improvement ‐ the odds ratio (OR) was used to measure treatment effect.
Overall estimates of the treatment difference are presented. In all cases the overall estimate from a fixed‐effects model is presented and a test for heterogeneity using a standard chi‐squared statistic performed. If there is significant heterogeneity a random‐effects model is presented. If a test of heterogeneity was negative then a weighted estimate of the typical treatment effect across trials was calculated. If, however, there was evidence of heterogeneity of the treatment effect between trials then either only homogeneous results were pooled, or a random‐effects model was used (in which case the confidence intervals would be broader than those of a fixed‐effects model).
Results
Description of studies
Forty‐one references were identified from the Cochrane Dementia and Cognitive Improvement Register of clinical trials and other sources as described above; none of these were new since the last update of this review in 2005. The pharmaceutical company Sigma‐Tau provided five additional references. Many of the trials have been supported by Sigma‐Tau. Of these thirteen studies were excluded on the basis of the abstract for the following reasons: one was a review of previous work with no new data; two were not randomized; three trials were single‐blind, one reported preliminary results only and three trials did not look at the population for consideration in this review. Three references were additional to other trials and are included under the primary reference accordingly.
The remaining thirty‐three citations were obtained and assessed. A further three were found to refer to other studies and were dealt with as above, and nineteen were excluded after assessment of the whole paper. (See table "Characteristics of excluded studies").
Update: the most recent search in May 2004 revealed four additional references of which one was a reference to a previously included trial (Spagnoli 1991) and one was a review of previous studies (Montgomery 2003). One study was excluded as it was single blind (Vecchi 1991). Costa 1993was added to included studies. Information from Sigma‐Tau included four new studies and additional data on five previously included studies. Please note that the study previously referred to as Livingston 1991 will now be referred to as James 1992as it is clear they refer to the same study.
Sixteen studies now fulfilled the inclusion criteria, of which fifteen provided sufficient data to be included in the meta‐analysis. ALC appeared to have no common or serious adverse side effects in most studies and a dose titration period was not required. Most studies used a dose of 2 g (range 1 to 3 g). All studies were placebo‐controlled.
All studies were parallel‐group design and subjects were randomly assigned to treatment or control groups. Six of the studies were multicentre. Trial size ranged from 30 to 431 subjects (average 115). The most common duration of treatment was 6 months (range 3 months to 1 year).
In more recent studies a diagnosis of dementia was made using accepted criteria such as DSM‐IV or NINCDS‐ADRDA (McKhann 1984). However for older studies DSM / NINCDS‐ADRDA criteria were not in common use. In order to ensure all relevant studies were included, criteria that seemed likely to include subjects with dementia were accepted e.g. Mantero 1989. Most trials included subjects with probable dementia or mild to moderate cognitive decline. Although this review was intended to assess ALC treatment in all dementia patients, all studies concentrated on Alzheimer's dementia and attempted to exclude other diagnoses. Depression was excluded using the Hamilton Rating Scale for Depression and the Hachinski Ischaemic Scale was used to exclude vascular dementia (excluding scores > 4). Update: James 1992 was included in which Hachinski Ischaemic Scale scores of < 7 were accepted. This raises the possibility that this study may have included subjects with vascular dementia and the results must be considered with this in mind.
Severity of disease was rated using Mini Mental State Examination (MMSE), the Blessed Dementia Scale (BDS, Spagnoli 1991) and the Global Deterioration Scale (Herrmann 1990)
Most trials recruited subjects aged over 60 years, except Spagnoli 1991 (over 40), Thal 1996 (over 50) and Bellagamba 1991(over 55).Thal 2000 specifically looked at an early onset group (45 to 65 years).
In all studies a variety of rating scales in the outcome areas of cognitive function, functional ability and severity of dementia were used. Global impression was assessed using the Clinical Global Impression (CGI) scale. This can be divided up into clinical global impression of change from baseline (CGI‐C), severity (CGI‐S) and efficacy (CGI‐E). Cognitive scales used included MMSE (eleven studies, although Sano 1992 used a modified version), Blessed Information Memory and Concentration Test (BIMC, five studies) and the ADAS‐cog (two studies). CGI was assessed in twelve studies. Assessment of functional ability was made using the Activities of Daily Living Scale (ADLS) in three studies. Herrmann 1990 used the Nuremberg Geriatric Daily Activities Scale (NAA), Rotmensch 1993 used the Nuremberg Gerontopsychological Self‐Rating Scale for Activities of daily Living (NAS). Testing of individual cognitive skills was performed in several studies and these tests included digit span, block tapping, verbal fluency, Rey's test, Corsi's test and the Toulouse‐Pieron test (five studies). Severity of dementia was measured using the Blessed Dementia Rating Scale (BDS, four studies), Clinical Dementia Rating Scale (CDR, two studies) and Sickness Impact Profile (SIP, one study).
In all studies the hypothesis was that ALC treatment would improve cognition or prevent decline in comparison with placebo in people with Alzheimer's disease.
Risk of bias in included studies
See Table "Characteristics of included studies"
Three studies described randomization adequately enough to be assigned 'A' status (James 1992; Rai 1990; Thal 2000). Update: with additional information a total of seven studies have been assigned 'A'. Drop‐outs were reported in all studies except Battistin 1989; Bellagamba 1991 and Passeri 1990. In most studies the drop‐outs were few, except Rai 1990. In this small study 44.4% (16/36) dropped out. Most of these drop‐outs were due to adverse effects of the drug. This high rate of adverse effects was not confirmed in much larger studies. All studies were described as double‐blind.
Effects of interventions
Sixteen trials were included in this analysis. All assessed the cognitive effects of ALC; in addition most considered severity of dementia, functional ability and clinical global impression. However, different studies considered different groups of outcomes. Nine trials reported an improvement in the treatment group in terms of cognitive measures (Battistin 1989; Bellagamba 1991; Costa 1993; James 1992; Mantero 1989; Onofrj 1992; Passeri 1990; Sano 1992; Spagnoli 1991). Two trials reported improvements in the MMSE (Mantero 1989; Onofrj 1992). Others found improvements in various subscales including verbal fluency (Battistin 1989), Rey's test (Bellagamba 1991) and digit span (Sano 1992; Rai 1990). Two large trials (Thal 1996; Thal 2000) did not find any statistically significant treatment effect on cognition. However in the 1996 study the authors suggested that in the early onset subgroup ALC may be beneficial. The second trial (Thal 2000) was designed specifically to test this hypothesis but was unable to confirm the previous findings. Mullin 1994 and Rai 1990 also did not find any differences between treatment and control groups. Rotmensch 1993 found that both groups improved over time in all areas. As they point out, this would be unexpected for patients with Alzheimer's dementia. They acknowledge this difficulty and suggest various explanations, such as the inexperience of researchers which may have allowed subjects with other diagnoses such as depression to be included. Results from this study are included, but the unusual results must be borne in mind when considering the data. We decided to include this study as, although the results are unexpected, there is no evidence of a systematic bias or other flaw in the methodology. We assume that any unknown factor will be affecting both placebo and treatment groups equally.
When considering Clinical Global impression six studies found differences in one or more subgroups when comparing treatment and placebo (Bayer 1994; Bellagamba 1991; Herrmann 1990; Mantero 1989; Onofrj 1992; Sano 1992). Three studies (James 1992; Thal 1996; Thal 2000) did not find a statistically significant treatment effect for clinical global impression.
Assessments of severity using the BDS reported treatment effect in favour of ALC in three trials (Passeri 1990; Mantero 1989; Spagnoli 1991). The groups using the SIP (Sano 1992) and CDR (Thal 1996; Thal 2000) found no statistically significant differences between treatment and placebo groups.
Using NAA Herrmann 1990 reported a statistically significant improvement in favour of ALC in patients self‐rating on daily activities. Assessments of functional behaviour using the ADLS (Thal 1996; Thal 2000) did not find any advantage for the treatment group.
It was observed that the included trials are of widely different durations (12 to 52 weeks). The results of the meta‐analysis are therefore presented according to trial duration.
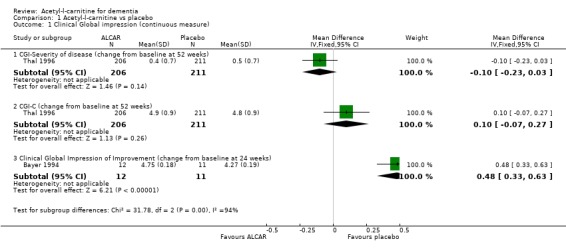
Two studies (Bayer 1994; Thal 1996) had data that could be entered into the analysis for CGI rating reported as a continuous variable. This did not reveal a statistically significant treatment effect on either Clinical Global Impression of Change (CGI‐C) (Weighted Mean Difference (WMD) 0.1, 95% Confidence Interval (CI) ‐0.07 to 0.27, P = 0.26) or Severity (CGI‐S) (WMD ‐0.1, 95% CI ‐0.23 to 0.03, P = 0.14) after 52 weeks. Bayer 1994 reported a significant positive effect for placebo when considering Clinical Global Impression of Improvement (CGI‐I) (WMD 0.48, 95% CI 0.33 to 0.63 P < 0.00001). When considering CGI‐I as a dichotomous variable (numbers improved versus numbers unchanged or worse) there were statistically significant treatment effects in favour of ALC at 12 and 24 weeks (OR 1.90, 95% CI 1.31 to 2.76) and (OR 2.33, 95% CI 1.31 to 4.14) but not at 52 weeks (OR 0.91, 95% CI 0.58 to 1.43). Clinical Global Impression of Severity and Efficacy were also considered as dichotomous variables. Severity was considered as normal‐mild versus moderate‐severe illness. Numbers showing an overall benefit were compared to numbers showing adverse effects or no benefit for efficacy. There were no significant results in these groups.
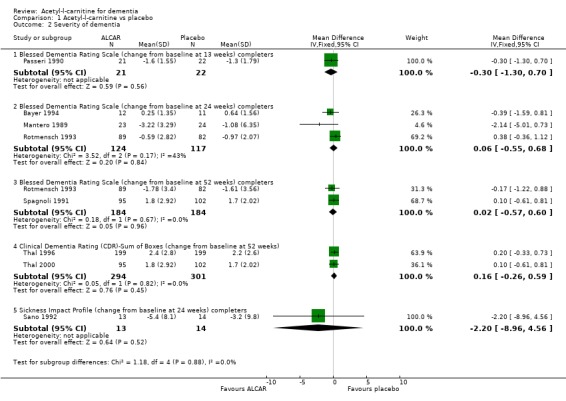
When considering the severity of dementia scales, again the results are presented according to different trial duration. It is conventional for a more positive score to reflect a better outcome (less severe dementia) and the direction of signs for the SIP results has been reversed to match this convention. Using the Blessed Dementia Rating Scale no significant difference was found between placebo and treatment groups (WMD ‐0.3, 95%CI ‐1.30 to 0.7, P = 0.56) over 13 weeks. Over 24 (WMD 0.06, 95% CI ‐0.55 to 0.68) and 52 (WMD 0.02, 95% CI ‐0.57 to 0.60, P = 0.96) weeks similarly, there was no statistically significant treatment effect. Thal 1996 used the Clinical Dementia Rating Scale ‐ Sum of Boxes in two studies over 52 weeks. There is no statistically significant treatment effect. Only one trial (Sano 1992) used the Sickness Impact Profile and analysis confirmed the authors' findings of no significant difference between placebo and treatment groups (WMD ‐2.2, 95% CI ‐8.96 to 4.56, P = 0.52).
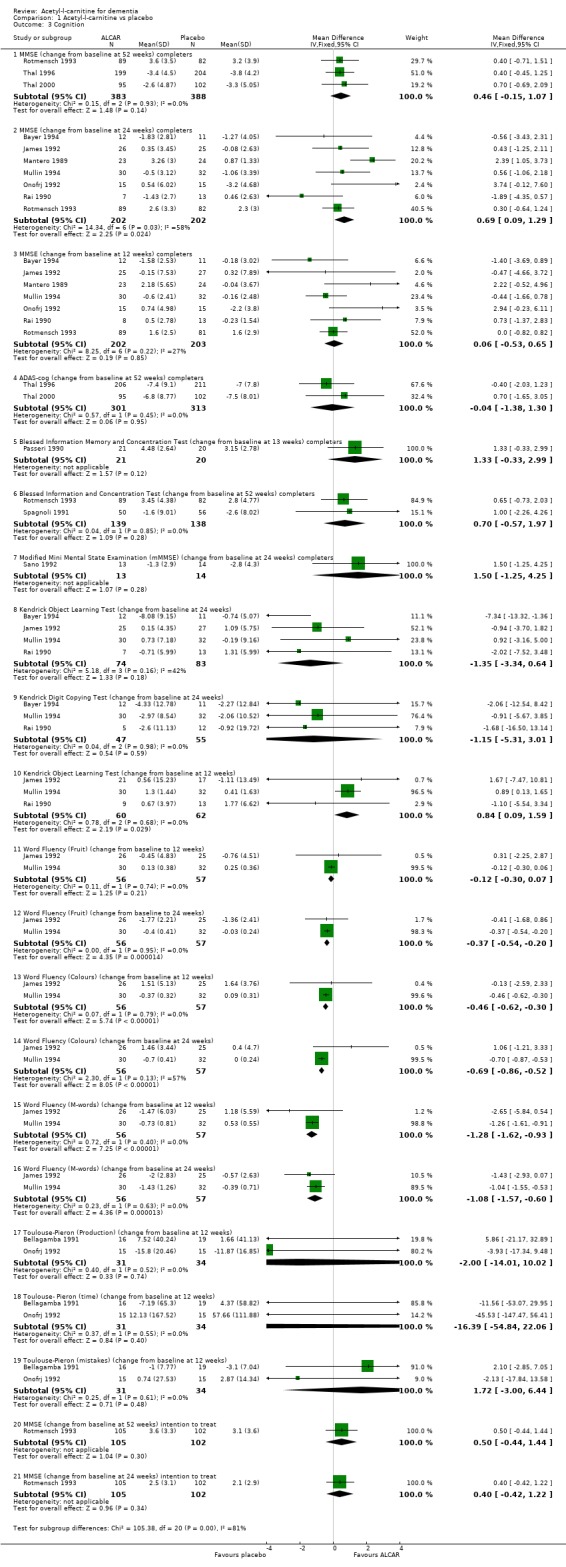
In the meta‐analysis of cognitive scales, studies using the MMSE did not show a statistically significant improvement on ALC compared with placebo over 12 weeks (WMD 0.06, 95% CI ‐0.53 to 0.65, P = 0.85) or 52 weeks (WMD 0.46, 95% CI ‐0.15 to 1.07, P = 0.14). However, there was a statistically significant treatment effect on MMSE at 24 weeks (WMD 0.69, 95% CI 0.09 to 1.29, P = 0.02). The signs for the ADAS‐cog results were reversed so that a more positive score is a better outcome. Again, the analysis did not reveal an advantage for the ALC treated group (WMD ‐0.04, 95%CI ‐1.38 to 1.30, P = 0.95). One trial (Passeri 1990) used the BIMC test over 13 weeks and there was no significant difference between placebo and treated groups (WMD 1.33, CI ‐0.33 to 2.99, P = 0.12). Other trials were of longer duration (Rotmensch 1993; Spagnoli 1991: 52 weeks) but this did not change the outcome (WMD 0.70, 95% CI ‐0.57 to 1.97, P = 0.28). Finally, Sano 1992 used a modified MMSE, and after 24 weeks there was no statistically significant treatment effect (WMD 1.50, 95% CI ‐1.25 to 4.25, P = 0.28). Many studies used a range of individual neuropsychological tests. However, they used different tests making it difficult to enter the results into an analysis. It was also felt that the results of an individual neuropsychological test would not be clinically relevant to the question of whether ALC is beneficial in dementia. Despite this, in the early stages of this update, several of these tests were analysed and none showed significant results, except for the Kendrick Object Learning Test at 12 weeks (WMD 0.84, 95% CI 0.09 to 1.59, P = 0.03). This effect did not persist to 24 weeks for the same test (WMD ‐1.35, 95% CI ‐3.34 to 0.64, P = 0.18). Other results for individual tests are not presented here, but would available on request.
All of the analyses used completers; however, in some studies intention to treat results were available. These results were analysed in a couple of cases; however, no significant differences were found between the results for completers and ITT, suggesting that this has not greatly affected overall conclusions. We looked at Rotmensch 1993 for MMSE at 52 weeks (WMD 0.5, 95% CI ‐0.44 to 1.44, P = 0.3) and 24 weeks (WMD 0.40, 95% CI ‐0.42 to 1.22, P = 0.34).
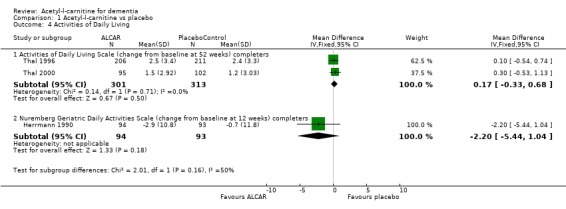
When considering functional ability, the results for the ADLS show no statistically significant difference between placebo and treatment group (WMD 0.17, 95%CI ‐0.33 to 0.68, P = 0.50). Herrmann 1990 used the NGDAS over 12 weeks and again no advantage for treatment was found (WMD ‐2.20, 95% CI ‐5.44 to 1.04, P = 0.18).
Drop‐outs from trials before completion were also analysed where they were reported. Total drop‐outs for any reason were not significantly different between the groups. Drop‐outs due to adverse events did not differ significantly between groups at 12 and 52 weeks. ALC appeared to have no common or serious adverse effects in most studies. The most common adverse effects that may have been related to treatment were gastrointestinal e.g. diarrhoea, nausea and vomiting. There were no statistically significant treatment effects for numbers of adverse events due to agitation, nausea, aggression, confusion, abdominal discomfort, total adverse events, nor for the numbers of deaths.
Discussion
This review considered the effect of ALC treatment in dementia. The use of multiple and differing assessment scales made comparisons difficult and therefore outcomes were simplified into several main areas. These were cognition, severity of dementia, functional ability and clinician impression.
It is clear that these studies are heterogeneous in several ways. Studies were of different durations, used different doses of ALC, slightly different age groups, different criteria for inclusion and differing assessment tools. We attempted to take this into account by using subgroup analysis. This heterogeneity should make one cautious in interpreting the results. In all analyses where more than one trial was included, a mathematical test of heterogeneity was performed. This is the case for all outcomes except CGI as a continuous variable. In all cases the source of possible error due to heterogeneity was not significant (P > 0.10), except when considering drop‐outs at 12 weeks (P = 0.14) and MMSE at 24 weeks (P = 0.03). The results for these outcomes should be interpreted with this in mind.
There are few available data on ALC pharmacokinetics in humans. ALC is a small molecule and can only be absorbed through an active transport mechanism. The acetyl group is easily removed both in the gastrointestinal tract and the liver. These mechanisms suggest that a large inter‐individual variability should be expected in the general population. Studies have not taken this into account in selecting the dose of ALC, and this may be a source of error.
There is some evidence that ALC has a positive effect on clinical impression (as measured by the CGI) when this is looked at as a binary measure (improved versus not improved). However, when so many comparisons are made some statistically significant results due to chance alone are to be expected, and ALC had no effect on CGI as a continuous measure.
Several of the earlier trials had suggested that ALC treatment might have beneficial effects on mental functioning, particularly memory. There was a statistically significant effect for ALC on MMSE at 24 weeks which was not present at 12 and 52 weeks. As pointed out above, there is a significant possibility that this result has been affected by the heterogeneity of the included trials. One must be cautious in attributing any clinical significance to this, especially as the effect is not sustained. There is no evidence for an effect on cognition from the meta‐analysis of results using other standardised cognitive scales.
Early trials also suggested there might be a positive effect on severity of dementia (as measured by the BDS). However the meta‐analysis did not show any significant effect of treatment with ALC on this outcome.
There is no evidence of significant adverse effects associated with ALC.
Of the studies that were methodologically acceptable, not all contained sufficient data to use in the meta‐analysis. At other times data were presented in such a way as to make them difficult to extract. This is particularly true of the earlier studies which tended to have more positive results. It is possible that this may have had an effect on the results and conclusions.
Update: the data provided by Sigma‐Tau allowed us to include several more trials in the meta‐analysis. This did not have any overall effect on the conclusions, but improves their reliability.
Authors' conclusions
Implications for practice.
The evidence of benefit of ALC for people with Alzheimer's disease does not justify recommending its routine clinical use.
Implications for research.
Although this review intended to assess all dementia groups, all included studies focused on Alzheimer's disease. Only one small, excluded, unpublished study examined vascular dementia but reported negative findings (Andrea Stracciari 2001 personal communication) so there may be scope for further trials in other types of dementia.
As discussed, there is a lack of data relating to ALC pharmacokinetics in humans and more research in this area is desirable. The criteria used for dose selection should be derived from a thorough oral bioavailability study that would guarantee a consistent increase in ALC plasma levels in all patients.
Most studies also concentrated on outcomes in the areas of cognition, severity of dementia and ability to perform activities of daily living. Further studies including other relevant outcomes such as mood and caregiver quality of life would be valuable in dementia. However, the evidence so far available does not suggest that ALC is likely to prove an important therapeutic agent for the treatment of people with dementia.
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 8 November 2007 | New search has been performed | 8 November 2007: an update search was run which did not identify any newly reported trials since this review was last updated in 2005. |
| 20 May 2005 | Amended | May 2005: Sigma Tau provided many additional company reports which allowed us to identify and include additional trials. The data provided by Sigma‐Tau in the reports allowed us to include these new trials in the meta‐analysis. This did not have any overall effect on the conclusions, but improves their reliability. |
| 21 February 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The reviewers gratefully acknowledge the help of lay editor Dolores Williams for her comments on the review. They also acknowledge the contribution of Antonella Bacchieri and Sigma‐Tau, and in particular the provision of large amounts of unpublished data which significantly contributed to the update of this review. The reviewers would also like to thank Jacqueline Birks for her assistance with statistical matters, Dymphna Hermans and Katherine Hicks of the Cochrane Dementia and Cognitive Improvement Group for all their help.
Data and analyses
Comparison 1. Acetyl‐l‐carnitine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Global impression (continuous measure) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 CGI‐Severity of disease (change from baseline at 52 weeks) | 1 | 417 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.23, 0.03] |
| 1.2 CGI‐C (change from baseline at 52 weeks) | 1 | 417 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.07, 0.27] |
| 1.3 Clinical Global Impression of Improvement (change from baseline at 24 weeks) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.33, 0.63] |
| 2 Severity of dementia | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Blessed Dementia Rating Scale (change from baseline at 13 weeks) completers | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.30, 0.70] |
| 2.2 Blessed Dementia Rating Scale (change from baseline at 24 weeks) completers | 3 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.55, 0.68] |
| 2.3 Blessed Dementia Rating Scale (change from baseline at 52 weeks) completers | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.57, 0.60] |
| 2.4 Clinical Dementia Rating (CDR)‐Sum of Boxes (change from baseline at 52 weeks) | 2 | 595 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.26, 0.59] |
| 2.5 Sickness Impact Profile (change from baseline at 24 weeks) completers | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐8.96, 4.56] |
| 3 Cognition | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 MMSE (change from baseline at 52 weeks) completers | 3 | 771 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.15, 1.07] |
| 3.2 MMSE (change from baseline at 24 weeks) completers | 7 | 404 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.09, 1.29] |
| 3.3 MMSE (change from baseline at 12 weeks) completers | 7 | 405 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.53, 0.65] |
| 3.4 ADAS‐cog (change from baseline at 52 weeks) completers | 2 | 614 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐1.38, 1.30] |
| 3.5 Blessed Information Memory and Concentration Test (change from baseline at 13 weeks) completers | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 1.33 [‐0.33, 2.99] |
| 3.6 Blessed Information and Concentration Test (change from baseline at 52 weeks) completers | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.57, 1.97] |
| 3.7 Modified Mini Mental State Examination (mMMSE) (change from baseline at 24 weeks) completers | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐1.25, 4.25] |
| 3.8 Kendrick Object Learning Test (change from baseline at 24 weeks) | 4 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐3.34, 0.64] |
| 3.9 Kendrick Digit Copying Test (change from baseline at 24 weeks) | 3 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐5.31, 3.01] |
| 3.10 Kendrick Object Learning Test (change from baseline at 12 weeks) | 3 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.09, 1.59] |
| 3.11 Word Fluency (Fruit) (change from baseline to 12 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.30, 0.07] |
| 3.12 Word Fluency (Fruit) (change from baseline to 24 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.54, ‐0.20] |
| 3.13 Word Fluency (Colours) (change from baseline at 12 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.62, ‐0.30] |
| 3.14 Word Fluency (Colours) (change from baseline at 24 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.86, ‐0.52] |
| 3.15 Word Fluency (M‐words) (change from baseline at 12 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐1.62, ‐0.93] |
| 3.16 Word Fluency (M‐words) (change from baseline at 24 weeks) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐1.57, ‐0.60] |
| 3.17 Toulouse‐Pieron (Production) (change from baseline at 12 weeks) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐14.01, 10.02] |
| 3.18 Toulouse‐ Pieron (time) (change from baseline at 12 weeks) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐16.39 [‐54.84, 22.06] |
| 3.19 Toulouse‐Pieron (mistakes) (change from baseline at 12 weeks) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.72 [‐3.00, 6.44] |
| 3.20 MMSE (change from baseline at 52 weeks) intention to treat | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.44, 1.44] |
| 3.21 MMSE (change from baseline at 24 weeks) intention to treat | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.42, 1.22] |
| 4 Activities of Daily Living | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Activities of Daily Living Scale (change from baseline at 52 weeks) completers | 2 | 614 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.33, 0.68] |
| 4.2 Nuremberg Geriatric Daily Activities Scale (change from baseline at 12 weeks) completers | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐5.44, 1.04] |
| 5 Dropouts before end of treatment | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Between baseline and 12 weeks | 3 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.66, 2.07] |
| 5.2 Between baseline and 24 weeks | 6 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 5.3 Between baseline and 52 weeks | 4 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.75, 1.38] |
| 6 Clinical Global Impression (improved vs no change/ worse) | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Clinical Global Impression of Improvement (change from baseline at 12 weeks) | 6 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.31, 2.76] |
| 6.2 Clinical Global Impression of improvement ( change from baseline at 24 weeks) | 6 | 233 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.31, 4.14] |
| 6.3 CIGIC (change from baseline at 52 weeks) | 2 | 338 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.58, 1.43] |
| 6.4 Clinical Global Impression of Severity at 24 weeks (normal‐mild versus mod‐severe illness) | 3 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.71, 2.20] |
| 6.5 Clinical Global Impression of Efficacy at 24 weeks) | 4 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.34, 1.02] |
| 6.6 Clinical Global Impression of Severity (normal/mild versus mod/severe) at 52 weeks | 1 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.70, 2.41] |
| 6.7 Clinical Global Impression of Efficacy (at 52 weeks) | 1 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.64] |
| 7 Dropouts due to adverse events | 11 | 1514 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.33] |
| 7.1 Between baseline and 12 weeks | 2 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.29, 1.53] |
| 7.2 Between baseline and 24 weeks | 5 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.47, 1.84] |
| 7.3 Between baseline and 52 weeks | 4 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.56, 1.84] |
| 8 Number suffering at least one adverse event | 10 | 1060 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.30] |
| 8.1 Between baseline and 12 weeks | 3 | 431 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.54, 1.70] |
| 8.2 Between baseline and 24 weeks | 8 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
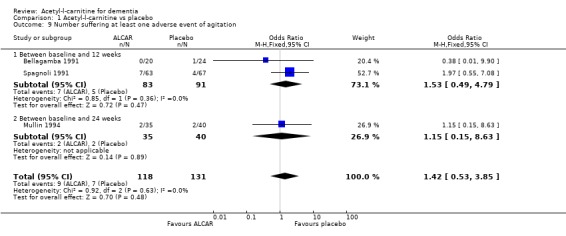
| 9 Number suffering at least one adverse event of agitation | 3 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.53, 3.85] |
| 9.1 Between baseline and 12 weeks | 2 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.49, 4.79] |
| 9.2 Between baseline and 24 weeks | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.15, 8.63] |
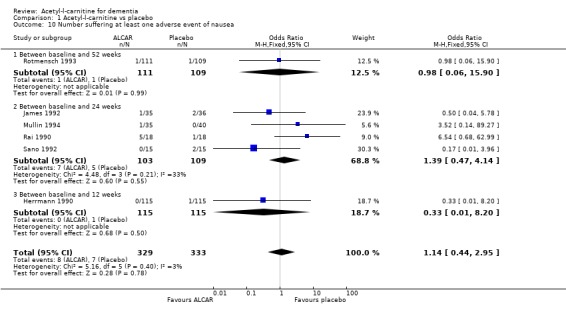
| 10 Number suffering at least one adverse event of nausea | 6 | 662 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.44, 2.95] |
| 10.1 Between baseline and 52 weeks | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.90] |
| 10.2 Between baseline and 24 weeks | 4 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.47, 4.14] |
| 10.3 Between baseline and 12 weeks | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
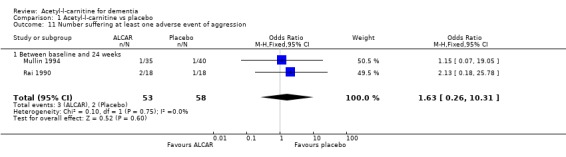
| 11 Number suffering at least one adverse event of aggression | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.26, 10.31] |
| 11.1 Between baseline and 24 weeks | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.26, 10.31] |
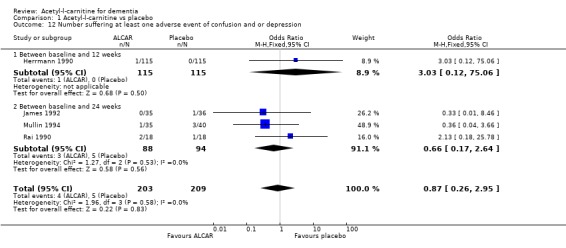
| 12 Number suffering at least one adverse event of confusion and or depression | 4 | 412 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.95] |
| 12.1 Between baseline and 12 weeks | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.06] |
| 12.2 Between baseline and 24 weeks | 3 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.17, 2.64] |
| 13 Number suffering at least one adverse event of abdominal discomfort or diarrhoea | 4 | 351 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.25, 2.76] |
| 13.1 Between baseline and 52 weeks | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.24, 9.07] |
| 13.2 Between baseline and 24 weeks | 3 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.77] |
| 14 Number of deaths | 8 | 1310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.52] |
| 14.1 Between baseline and 12 weeks | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
| 14.2 Between baseline and 24 weeks | 4 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.24, 4.80] |
| 14.3 Between baseline and 52 weeks | 3 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.20, 1.67] |
1.1. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 1 Clinical Global impression (continuous measure).
1.2. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 2 Severity of dementia.
1.3. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 3 Cognition.
1.4. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 4 Activities of Daily Living.
1.5. Analysis.
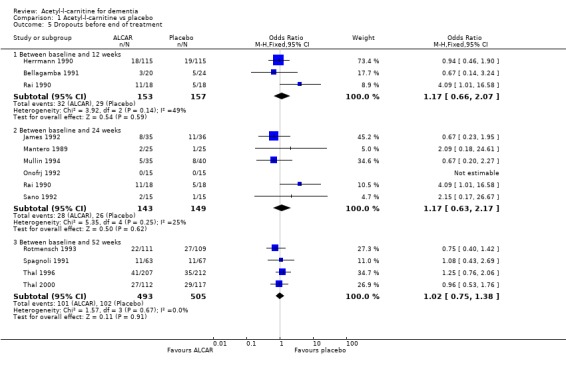
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 5 Dropouts before end of treatment.
1.6. Analysis.
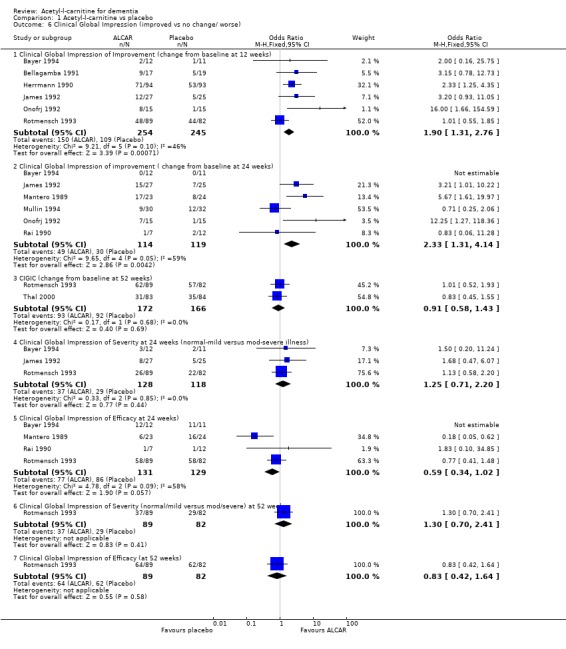
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 6 Clinical Global Impression (improved vs no change/ worse).
1.7. Analysis.
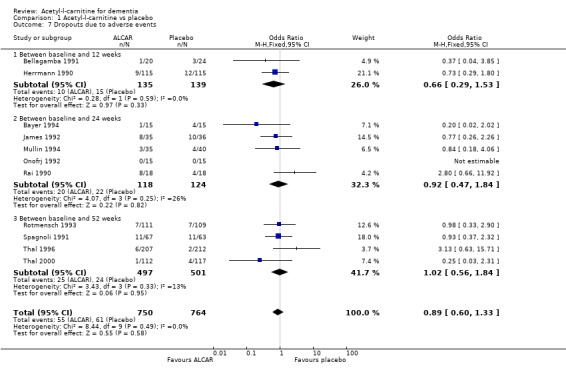
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 7 Dropouts due to adverse events.
1.8. Analysis.
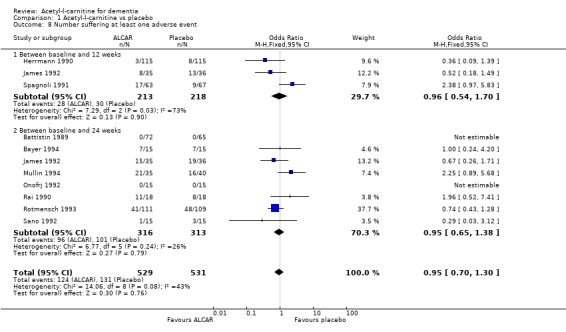
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 8 Number suffering at least one adverse event.
1.9. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 9 Number suffering at least one adverse event of agitation.
1.10. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 10 Number suffering at least one adverse event of nausea.
1.11. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 11 Number suffering at least one adverse event of aggression.
1.12. Analysis.
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 12 Number suffering at least one adverse event of confusion and or depression.
1.13. Analysis.
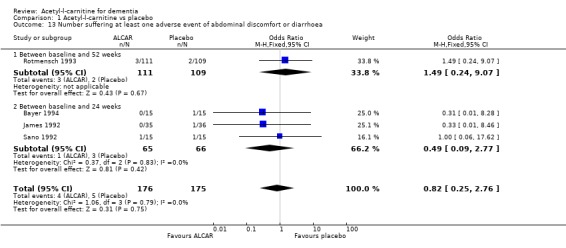
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 13 Number suffering at least one adverse event of abdominal discomfort or diarrhoea.
1.14. Analysis.
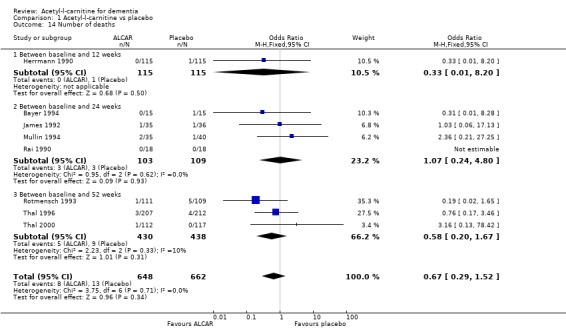
Comparison 1 Acetyl‐l‐carnitine vs placebo, Outcome 14 Number of deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battistin 1989.
| Methods | Multi centre, randomized, double blind placebo controlled study | |
| Participants | Country: Italy Multicentre Selection criteria: Probable Alzheimer's Disease (AD) as specified by NINCDS‐ADRDA criteria Mean age : subjects 69.8+/‐8.5y, controls 71.1+/‐ 8.0 137 patients (91 males, 46 females) | |
| Interventions | 1.ALC 2g/day for 180 days 2. Placebo for 180 days | |
| Outcomes | Digit span, block tapping, verbal fluency, copying test, MMSE, BDS, BIMC, Raven's matrices, digit symbol, Rey's test | |
| Notes | Methodology briefly described | |
Bayer 1994.
| Methods | Randomized, double blind, placebo controlled study | |
| Participants | Country: Wales Selection criteria: mild Alzheimer Type dementia, MMSE 12‐22, HIS < 4. Age range 61‐87, 11 male, 19 female | |
| Interventions | 1. ALC 2g/day for 24 weeks 2. Placebo for 24 weeks | |
| Outcomes | MMSE, HRSD, BDS, GBS, KOLT, KDCT, CGI‐S, CGI‐C, CGI‐E, Relatives Assessment | |
| Notes | Analysis of completers, used replacements, unclear if this affects results. Data from S‐T files | |
Bellagamba 1991.
| Methods | Randomized, parallel, double blind, placebo controlled study | |
| Participants | Country: Italy Selection criteria: diagnosis of dementia made clinically, HIS < 4, RAVLT short term > 10 < 40, RAVLT long term < 6 | |
| Interventions | 1. ALC 3g/day 12 weeks 2. Placebo 12 weeks | |
| Outcomes | SCAG, RAVLT, Raven Progressive Matrices, Toulouse‐Pieron, GBS, CGI‐C | |
| Notes | Data from S‐T files | |
Costa 1993.
| Methods | Randomized, parallel, double blind, placebo controlled study | |
| Participants | Country: Italy Selection criteria: probable dementia Alzheimers type according to DSM III‐R and NINCDS‐ADRDA criteria. MMSE 16‐24,GDS 4‐5 Exclusion criteria Hamilton Rating scale >18 | |
| Interventions | 1. ALC 2g/day for 16 weeks 2. Placebo for 16 weeks | |
| Outcomes | Reys test, Digit span, Corsi's test, digit symbol test, Gibson's spiral, verbal fluency, GBS | |
| Notes | ||
Herrmann 1990.
| Methods | Randomized double blind placebo controlled study | |
| Participants | Country: Germany Multicentre Selection criteria: mild to moderate cognitive decline corresponding to stages 3 or 4 on the Global Deterioration Scale (80% GDS stage 3) 230 male and female patients age 60‐80 years | |
| Interventions | 1. ALC 1.5g/day for 12 weeks 2. Placebo for 12 weeks | |
| Outcomes | CGI‐C, NGDAS, modified digit symbol substitution test. | |
| Notes | Analysed completers, not intention to treat | |
James 1992.
| Methods | Randomized, double blind, placebo controlled study | |
| Participants | Country: UK Single centre Selection criteria: clinical diagnosis of mild‐moderate dementia, HIS < 7, impaired score on KOLT | |
| Interventions | 1. ALC 2g/day for 24 weeks 2. placebo for 24 weeks | |
| Outcomes | MMSE, Names Learning Test, Word Fluency Test, Drawing Test, Recognition memory for Words and pictures, Geriatric Mental State Assessment, CGI‐C, CGI‐S, Performance of ADLs, KOLT | |
| Notes | Analysed completers, from S‐T data. Some patients with depression included, but not severe. | |
Mantero 1989.
| Methods | Randomization method not described. Double blind, placebo controlled study | |
| Participants | Country: Italy Single centre Selection criteria: MMSE < 24, Hachinski's Ischaemic score < 4, HRSD Age range 60‐97, 21 males, 29 females | |
| Interventions | 1. ALC 2g/day for 180 days 2. placebo for 24 weeks | |
| Outcomes | MMSE, BDS, BIMC, HRSD, CGI‐C, CGI‐S, CGI‐E, HIS | |
| Notes | Little information on adverse events | |
Mullin 1994.
| Methods | Randomized using tables of random numbers, concealed, double blind placebo controlled, parallel study | |
| Participants | Country: UK Single centre Selection criteria: Mild AD, clinically diagnosed and using Global Deterioration Scale, HIS <4 Age range 62‐95 M 20 F 42 | |
| Interventions | 1. ALC 2g/day for 180 days 2. placebo for 180 days | |
| Outcomes | MMSE, CGI, GDS, NART, KOLT, Kendrick Digit copying Test, Names Learning test, ADL, Word Fluency | |
| Notes | High numbers of adverse events in treatment group | |
Onofrj 1992.
| Methods | Randomized, method not described, double blind, placebo controlled | |
| Participants | Country: Italy Single centre Selection criteria: HRSD < 14, HIS < 4, MMSE 10‐23, GDS 3‐5 | |
| Interventions | 1. ALC 3g for 180 days 2. Placebo for 180 days | |
| Outcomes | MMSE, Raven's matrices, RGDS, T‐P, verbal span fluency, SCAG, Set test, Buschke Memory test, visual detention test, GSBS, CGI‐C | |
| Notes | ||
Passeri 1990.
| Methods | Randomization method not described. Placebo controlled double blind trial | |
| Participants | Country: Italy Single centre Selection criteria: DSM III criteria for dementia, HIS <6 Age range: subjects mean age 74 +/‐ 6.1, controls 75.1 +/‐ 5.30 20 males, 38 females | |
| Interventions | 1. ALC 2g/day for 90 days 2. Placebo for 90 days | |
| Outcomes | MMSE, SHGRS, BDS, BIMC, Rey's test, Corsi's test, verbal fluency, Toulouse‐ Pieron, digit span, HDRS, Gibson's test | |
| Notes | ||
Rai 1990.
| Methods | Randomization was made using a predetermined code. Double blind, placebo controlled trial | |
| Participants | Country: UK Single centre Selection criteria: mild‐ moderate AD on Global deterioration scale, HIS <4 Mean age: 79 10 males, 26 females | |
| Interventions | 1. ALC 2g/day for 180 days 2. Placebo for 180 days | |
| Outcomes | GDS, RGDS, KOLT, KDCT,ADL, CGA, MMSE, CGI‐C, CGI‐E, word fluency test | |
| Notes | Additional unpublished data obtained from Sigma Tau , CGI scores given but no SDs | |
Rotmensch 1993.
| Methods | Randomised using permutation blocks. Double blind, parallel, placebo controlled trial | |
| Participants | Country: Germany, multicentre, Selection criteria: mild‐moderate Alzheimers disease, HIS <4, MMSE 13‐23, NINCDS‐ADRDA criteria. | |
| Interventions | 1. ALC 3g/day for 1 year 2. Placebo for 1 year | |
| Outcomes | MMSE, BIMC, CGI‐C, CGI‐E, CGI‐S, NAS, BDS, RGDS | |
| Notes | Data from Sigma‐Tau, unpublished. Unusual results‐ improvement over one year. Not usual clinical course for dementia. Raises issue of incorrect diagnosis. | |
Sano 1992.
| Methods | Randomization mentioned but not described. Double blind placebo controlled study | |
| Participants | Country: USA Single centre Selection criteria: dementia according to NINCDS‐ADRDA Age Range: 60‐80 30 patients | |
| Interventions | 1. ALC 2.5g/day for 90 days followed by ALC 3g/day for 90 days 2. placebo for 180 days | |
| Outcomes | mMMSE, CGI‐S, CGI‐C, verbal fluency, SIP, SMQ, Selective reminding test, Benton visual retention test, Wechsler memory scale, digit span | |
| Notes | CGI results not reported | |
Spagnoli 1991.
| Methods | Subjects were randomised in blocks of four according to a centrally prepared randomisation list. Double blind, placebo controlled study | |
| Participants | Country: Italy Multicentre Selection criteria: Diagnosis of dementia syndrome following DSM III criteria, modified HIS < 4 Age range: over 40 years males 38, females 92 | |
| Interventions | 1. ALC 2g/day for 1 year 2. Placebo for 1 year | |
| Outcomes | SBI, BDS, BIMC, Raven's matrices, prose memory, apraxia, finger agnosia, | |
| Notes | ||
Thal 1996.
| Methods | Subjects were randomized using lists. Placebo‐controlled double blind trial | |
| Participants | Country: USA Multicentre Selection criteria: Probable AD according to NINCDS/ADRDA criteria, DSM III, MSE 13‐26, HIS < 4 Age range:50 years and above 183 males, 248 females | |
| Interventions | 1. ALC 3g/day over 1 year 2. Placebo for 1 year | |
| Outcomes | ADAS ‐cog, ADAS non‐cog, CDR, ADL, IADL, CGI‐S, CGI‐C, MMSE | |
| Notes | ||
Thal 2000.
| Methods | Randomized using permuted block design. Double‐blind placebo controlled trial | |
| Participants | Country: USA Multicentre Selection criteria: Probable AD according to NINCDS/ADRDA criteria, DSM III, MSE 13‐26, HIS <4 Age range: 45‐65 119 males, 108 females | |
| Interventions | 1. ALC 3g/day for 1 year 2. Placebo for 1 year | |
| Outcomes | ADAS ‐cog, ADAS non‐cog, CDR, ADL, MMSE, CBIC | |
| Notes | Early onset only, used modified intention to treat analysis | |
ADAS: Alzheimers Disease Assessment scale (cognitive and non‐cognitive subscales), CDR: clinical dementia rating scale, ADL: activities of daily living, IADL: instrumental activities of daily living, CGI‐S: clinical global impression of severity, CGI‐C:change/improvement, CGI‐E: efficacy, MMSE: mini‐mental state examination (mMMSE: modified), CBIC: clinician based impression of change, SBI: spontaneous behaviour interview, BDS: Blessed dementia scale, BIMC: Blessed information and memory concentration test, SIP: Sickness Impact Questionnaire, SMQ: Squires memory questionnaire, RM: recognition memory, SHGRS: Stuard Hospital geriatric rating scale, GDS: Geriatric Depression Scale, HRSD: Hamilton Rating Scale for Depression, HIS: Hachinski Ischaemic Score, NGDAS: Nuremberg Geriatric Daily Activities Scale, SCAG: Sandoz Clinical Assessment‐ Geriatric, RAVLT: Reys Auditory Verbal Learning Test, T‐P: Toulouse‐Pieron, GSBS: Geriatric Scale of Behavioural and Self Suffiency Evaluation, KOLT: Kendrick Object Learning Test, KDCT: Kendrick Digit Copying Test, GBS: Gottfries‐Brane‐Steen Scale, RGDS: Reisberg Global Deterioration Scale,NAS: Nuremberg Gerontopsychological Self‐Rating Activities of Daily Living Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acierno 1983 | Not randomized. |
| Amaducci 1990 | Review of trials. |
| Arrigo 1988 | This trial looked at cerebrovascular insufficiency, not dementia. |
| Arrigo 1990 | This trial looked at cerebrovascular insufficiency, not dementia. |
| Bertolino 1983 | Double blind placebo controlled trial, not randomized. |
| Bonavita 1986 | Subjects had 'senile brain' not clearly diagnosed dementia. |
| Bowman 1992 | Review of trials. |
| Bravi 1994 | Results of meta analysis of previous studies. |
| Calvani 1992 | Review of trials. |
| Campi 1990 | Single‐blind study with no placebo arm. |
| Carta 1991 | Review of data. |
| Cipolli 1990 | Preliminary results of Salvioli 1994, single‐blind trial. |
| Frattola 1991 | Subjects allocated to treatment group according to a predetermined sequence, not randomized. |
| Garzya 1990 | This trial considered depressed subjects, not the population under consideration. |
| Genazzani 1990 | This was not a randomized double‐blind trial and had no extractable data. |
| Giuliani 1990 | Double‐blind placebo controlled trial but not randomized |
| Goetz 1990 | Subjects with Huntington's disease, not the population under consideration. |
| Montgomery 2003 | Review and meta‐analysis of previous studies |
| Parnetti 1993 | Multicentre randomized study comparing ALC with l‐alpha‐glyceryl‐phosphorylcholine, no placebo arm. |
| Passeri 1988 | Subjects had 'mild mental impairment' , did not fulfil criteria for a diagnosis of dementia. |
| Pettegrew 1995 | Study not randomized. |
| Salvioli 1994 | Single‐blind multicentre trial comparing ALC with placebo, serial design. |
| Sinforiani 1990 | Single‐blind study. |
| Stracciari 1988 | Unpublished trial, data not available. |
| Tomasina 1987 | Not randomized or blinded, no placebo control group. |
| Vecchi 1991 | Single‐blind trial comparing ALC to placebo, serial design. |
Characteristics of ongoing studies [ordered by study ID]
Calvani 1998.
| Trial name or title | Acetyl‐l‐carnitine in the treatment of Alzheimer's disease patients |
| Methods | |
| Participants | Alzheimer's disease patients |
| Interventions | 14 intravenous infusion of ALC followed by 6 month oral treatment |
| Outcomes | 1.Cholinomimetic activity after 14 day infusion period 2. ALC efficacy after 6 months oral treatment |
| Starting date | Due to start soon after July 1998 |
| Contact information | fermina.orlandi@sigma‐tau.it |
| Notes |
Contributions of authors
‐SH: all correspondence on Review, drafting of Review, searches, selection and assessment of studies, updates, input in RevMan, data analysis ‐NT: selection of studies, drafting review verisons
‐Contact editor: Mario Fioravanti ‐Consumer editor: Owain Bennallack ‐The review has been peer reviewed anonymously by two peer reviewers
Sources of support
Internal sources
Maudsley Hospital, UK.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Battistin 1989 {published data only}
- Battistin L, Pizzolato G, Dam M, Ponza I, Perlotto N, Bergamo LC, Furlanut M De, Ziliotto R, Bardin G, Chinaglia L, Ferro Milione F, Lorizio A, Luria E, Ravenna C, Toso V, Zanetti R, Bendetti N, Calvani M. Effects of acetyl‐L‐carnitine (ALC) treatment in dementia: A multicentric, randomized, double‐blind study. New Trends in Clinical Neuropharmacology 1989;3(2):131‐2. [Google Scholar]
Bayer 1994 {unpublished data only}
- Bayer. The Effect of Acetyl‐l‐Carnitine in Senile Dementia of the Alzheimer Type. On file Sigma Tau.
Bellagamba 1991 {unpublished data only}
- Bellagamba G. Efficacy of Acetyl‐l‐carnitine in patients with degenerative dementia (SDAT)/Statistical Report. On file Sigma Tau.
- Bellagamba G, Postacchini D, Moretti V, Pennacchietti L. Acetyl‐l‐carnitine activity in senile dementia Alzheimer type. Neurobiology of Aging 1990;11:345. [Google Scholar]
Costa 1993 {published data only}
- Costa A, Martignoni E, Bono G, Sinforiani E, Petraglia F, Genazzani A, Nappi G. Pituitary‐adrenal function and cognitive performance in demented patients on acetyl‐l‐carnitine treatment. Medical Sciences Research 1993;21:589‐91. [Google Scholar]
Herrmann 1990 {published data only}
- Herrmann. Demonstration of the Effects of Acetyl‐l‐carnitine on impaired brain functions in geriatric outpatients. on file Sigma‐ Tau..
- Herrmann WM, Dietrich B, Hiersemenzel R. Pharmaco‐electroencephalographic and clinical effects of the cholinergic substance‐‐acetyl‐L‐carnitine‐‐in patients with organic brain syndrome. International Journal of Clinical Pharmalogical Research 1990;10(1‐2):81‐4. [PubMed] [Google Scholar]
- Herrmann WM, Stephan K. Efficacy and clinical relevance of cognition enhancers. Alzheimer's disease and associated disorders 1991;5(Supplement 1):7‐12. [DOI] [PubMed] [Google Scholar]
James 1992 {published and unpublished data}
- James I. M. The Effect of Acetyl‐l‐carnitine in senile dementia of Alzheimer type. on file Sigma‐Tau.
- Livingston GA, Sax KB, McClenahan Z, Blumenthal E, Foley K, Willison J, et al. Acetyl‐L‐carnitine in dementia. International Journal of Geriatric Psychiatry 1991;6(12):853‐60. [Google Scholar]
Mantero 1989 {published data only}
- Mantero. Effects of Acetyl‐l‐carnitine treatment in geriatric patients suffering from mental deterioration. on file Sigma‐Tau..
- Mantero MA, Barbero M, Giannini R, Grosso VG, Tomasina C, Iannuccelli M. Acetyl‐L‐carnitine as a therapeutic agent for mental deterioration in geriatric patients. (Double‐blind placebo controlled study). New Trends in Clinical Neuropharmacology 1989;3(1):17‐24. [Google Scholar]
Mullin 1994 {unpublished data only}
- Mullin. The Effect of Acetyl‐l‐carnitine in Senile Dementia of the Alzheimer Type. on file Sigma‐Tau.
Onofrj 1992 {unpublished data only}
- Onofrj M. Evaluation of Efficacy of chronic administration (6 months) of Acetyl‐l‐carnitine in patients suffering from Senile Dementia Alzheimer Type (SDAT) and ALzheimer Disease (AD). on file Sigma‐Tau.
Passeri 1990 {published data only}
- Cucinotta D, Passeri M, Ventura S, Ianuccelli M, Senin U, Bonati PA, Parnetti L. Multicenter clinical placebo controlled study with acetyl‐l‐carnitine (LAC) in the treatment of mildly demented elderly patients. Drug Development Research 1988;14(3‐4):213‐216. [Google Scholar]
- Passeri M, Cucinotta D, Bonati PA, Iannuccelli M, Parnetti L, Senin U. Acetyl‐L‐carnitine in the treatment of mildly demented elderly patients. International Journal of Clinical Pharmalogical Research 1990;10(1‐2):75‐9. [PubMed] [Google Scholar]
- Passeri M, Iannuccelli M, Ciotti G, Bonati P A, Nolfe G, Cucinotta D. Mental Impairment in Aging: Selection of patients, methods of evaluation and therapeutic possinilites of Acetyl‐l‐carnitine. International Journal of Clinical Pharmacological Research 1988;8(5):367‐376. [PubMed] [Google Scholar]
Rai 1990 {published data only}
- Rai G, Exton‐Smith AN, Wright G, Scott, Beston B. Double blind placebo controlled parallel group study of the efficacy and tolerability of Acetyl‐l‐carnitine in Elderly patients with Mild to Moderate dementia of Alzheimers type. On file at Sigma Tau 1989.
- Rai G, Wright G, Scott L, Beston B, Rest J, Exton‐Smith AN. Double‐blind, placebo controlled study of acetyl‐l‐carnitine in patients with Alzheimer's dementia. Current Medical Research and Opinion 1990;11(10):638‐47. [DOI] [PubMed] [Google Scholar]
Rotmensch 1993 {unpublished data only}
- Rotmensch. Efficacy and Safety of Acetyl‐l‐carnitine versus Placebo upon lon term treatment in elderly patients with Senile Dementia Alzheimer Type. On file Sigma Tau.
Sano 1992 {published data only}
- Sano M, Bell K, Cote L, Dooneief G, Lawton A, Legler, Marder K, Niani A, Stern Y, Mayeux R. Double‐blind parallel design pilot study of acetyl levocarnitine in patients with Alzheimer's disease. Archives of Neurology 1992;49(11):1137‐1141. [DOI] [PubMed] [Google Scholar]
Spagnoli 1991 {published data only}
- Lucca U, Menasce G, Comelli M, Cizza G, Bressi S, Frattura L, et al. An example of a clinical trial in patients with Alzheimer's disease: some methodological issues. Alzheimer's Disease and Associated Disorders 1989;317:1301‐10. [PubMed] [Google Scholar]
- Spagnoli A, Comelli M, Lucca U, Menasce G. L‐acetyl carnitine and Alzheimer's disease: results of a controlled clinical trial. Proceedings of the 6th international Stockholm/Springfield Symposium on Advances in ALzheimer Therapy 1990;11(3):342‐3. [Google Scholar]
- Spagnoli A, Lucca U, Menasce G, Bandera L, et al. Long‐term acetyl‐L‐carnitine treatment in Alzheimer's disease. Neurology 1991;41(11):1726‐32. [DOI] [PubMed] [Google Scholar]
Thal 1996 {published data only}
- Brooks III JO, Yesavage JA, Carta A, Bravi D. Acetyl‐L‐carnitine slows decine in younger patients with Alzheimer's disease: A reanalysis of a double blind, placebo controlled study using the trilinear approach. International Psychogeriatrics 1998;10(2):193‐203. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Carta A, Clarke WR, Ferris SH. A 1‐year multicenter placebo‐controlled study of acetyl‐L‐carnitine in patients with Alzheimer's disease. Neurology 1996;47(3):705‐11. [DOI] [PubMed] [Google Scholar]
Thal 2000 {published data only}
- Barrett AM, Thal L J. A 1 year controlled trial of acetyl‐l‐carnitine in early onset Alzheimer's disease. Neurology 2001;56(3):425. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Calvani M, Amato A, Carta A. A 1‐year controlled trial of acetyl‐l‐carnitine in early‐onset AD. Neurology 2000;55(6):805‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acierno 1983 {published data only}
- Acierno G. The use of 1‐acetylcarnitine in (presenile and senile) Alzheimer's disease. Preliminary results. Clinica Terapeutica 1983;105(2):135‐45. [PubMed] [Google Scholar]
Amaducci 1990 {published data only}
- Amaducci L, Lippi A. The dementias [Le Demenze]. Medicina Firenze 1990;10(3):213‐27. [PubMed] [Google Scholar]
Arrigo 1988 {published data only}
- Arrigo A, Clano E, Casale R, Buonocore M. The effects of L‐acetylcarnitine on reaction times in patients with cerebrovascular insufficiency. A double blind cross‐over study. Clinical Trials Journal 1988;25(SUPPL. 1):47‐56. [PubMed] [Google Scholar]
Arrigo 1990 {published data only}
- Arrigo A, Casale R, Buonocore M, Ciano C. Effects of acetyl‐L‐carnitine on reaction times in patients with cerebrovascular insufficiency. International Journal of Clinical Pharmalogical Research 1990;10(1‐2):133‐7. [PubMed] [Google Scholar]
Bertolino 1983 {published data only}
- Bertolino A, G Papagno. [Attivita della L‐Acetil Carnitina nel deterioramento mentale]. Clinica Europea 1983:384‐395. [Google Scholar]
Bonavita 1986 {published data only}
- Bonavita E. Study of the efficacy and tolerability of L‐acetylcarnitine therapy in the senile brain. INTERNATIONAL JOURNAL OF CLINICAL PHARMACOLOGY THERAPY AND TOXICOLOGY 1986;24(9):511‐6. [PubMed] [Google Scholar]
Bowman 1992 {published data only}
- Bowman BAB. Acetyl‐carnitine and Alzheimer's disease. Nutrition Reviews 1992;50(5):142‐4. [DOI] [PubMed] [Google Scholar]
Bravi 1994 {unpublished data only}
- Bravi D, Koch G, Calvani M, Bacchieri A, Pola P, NiBhuachalla S, Carta A. A meta‐analysis evaluation of clinical trials in Alzheimer's Patients treated with Acetyl‐l‐carnitine. Society for Neuroscience Abstracts. 1991; Vol. 20:1778.
Calvani 1992 {published data only}
- Calvani M, Carta A, Caruso G, Benedetti N, Iannuccelli M. Action of acetyl‐L‐carnitine in neurodegeneration and Alzheimer's disease. Annals of the New York Academy of Sciences 1992;663:483‐6. [DOI] [PubMed] [Google Scholar]
Campi 1990 {published data only}
- Campi N, Todeschini GP, Scarzella L. Selegiline versus L‐acetylcarnitine in the treatment of Alzheimer‐type dementia. Clinical Therapeutics 1990;12(4):306‐14. [PubMed] [Google Scholar]
Carta 1991 {published data only}
- Carta A, Calvani M. Acetyl‐L‐carnitine: a drug able to slow the progress of Alzheimer's disease?. Annals of the New York Academy of Sciences 1991;640:228‐32. [DOI] [PubMed] [Google Scholar]
Cipolli 1990 {published data only}
- Cipolli C, Chiari G. Effects of L‐acetylcarnitine on mental deterioration in the aged: initial results. Clinica Terapeutica 1990;132(6 Suppl):479‐510. [PubMed] [Google Scholar]
Frattola 1991 {unpublished data only}
- Bassi S, Ferrarese C, Finoia M. L‐Acetylcarnitine in Alzheimers Disease and senile dementia Alzheimer Type. In: Agnioli A, Cahn J, Lassen N, Mayeux R editor(s). Senile Dementia. J. Libbey, 1988:461‐66. [Google Scholar]
- Frattola. Sigma Tau 1988.
Garzya 1990 {published data only}
- Garzya G, Corallo D, Fiore A, Lecciso G, Petrelli G, Zotti C. Evaluation of the effects of L‐acetylcarnitine on senile patients suffering from depression. Drugs Under Experimental and Clinical Research 1990;16(2):101‐6. [PubMed] [Google Scholar]
Genazzani 1990 {published data only}
- Genazzani E. A controlled clinical study on the efficacy of L‐acetylcarnitine in the treatment of mild‐to‐ moderate mental deterioration in the aged. Conclusions. Clinica Terapeutica 1990;132(6 Suppl):511‐22. [PubMed] [Google Scholar]
Giuliani 1990 {published data only}
- Giuliani A, Calvani M, et al. XVII Congress of Collegium Internationale Neuro‐Psychopharmacologicum, Kyoto. 1990.
Goetz 1990 {published data only}
- Goetz CG, Tanner CM, Cohen JA, Thelen JA, Carroll VS, Klawans HL, et al. L‐acetyl‐carnitine in Huntington's disease: double‐blind placebo controlled crossover study of drug effects on movement disorder and dementia [letter]. Movement Disorders 1990;5(3):263‐5. [DOI] [PubMed] [Google Scholar]
Montgomery 2003 {published data only}
- Montgomery SA, Thal L.J, Amrein R. Meta‐analysis of double blind randomized controlled clinical trials acetyl‐l‐carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. International Clinical Psychopharmacology 2003;18:61‐71. [DOI] [PubMed] [Google Scholar]
Parnetti 1993 {published data only}
- Parnetti L, Abate G, Bartorelli L, Cucinotta D, Cuzzupoli M, Maggioni M, Villardita C, Senin U. Multicentre study of l‐alpha‐glyceryl‐phosphorylcholine vs ST200 among patients with probable senile dementia of Alzheimer's type. Drugs and Aging 1993;3(2):159‐64. [DOI] [PubMed] [Google Scholar]
Passeri 1988 {published data only}
- Passeri M, Iannuccelli M, Ciotti G, Bonati PA, Nolfe G, Cucinotta D. Mental impairment in aging: selection of patients, methods of evaluation and therapeutic possibilities of acetyl‐L‐carnitine. International Journal of Clinical Pharmalogical Research 1988;8(5):367‐76. [PubMed] [Google Scholar]
Pettegrew 1995 {published data only}
- Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl‐L‐carnitine in Alzheimer's disease. Neurobiology of Aging 1995;16(1):1‐4. [DOI] [PubMed] [Google Scholar]
Salvioli 1994 {published data only}
- Salvioli G, Neri M. L‐acetylcarnitine treatment of mental decline in the elderly. Drugs Under Experimental and Clinical Research 1994;20(4):169‐76. [PubMed] [Google Scholar]
Sinforiani 1990 {published data only}
- Sinforiani E, Iannuccelli M, Mauri M, Costa A, Bono G, Nappi G. Neuropsychological changes in demented patients treated with acetyl‐l‐carnitine. International Journal of Clinical Pharmacology Research 1990;10(1‐2):69‐74. [PubMed] [Google Scholar]
Stracciari 1988 {published data only (unpublished sought but not used)}
- Stracciari A, Ciucci G, Loreta R, Benedetti N, Ianuccelli M. Multi‐ infarct dementia: clinical trial with acetyl‐l‐carnitine vs placebo. Journal of Neurology 1988; Vol. 92.
Tomasina 1987 {published data only}
- Tomasina C, Barbero M, Mantero M, Manzino M, Pastorino P. Clinical, neuropsychological and electrophysiological assessment of the effects of L‐acetyl‐l‐carnitine in patients with mental deterioration [Valutazione clinica, neuropsicologia ed elettrofisiologica degli effetti della L‐acetilcarnitina in pazienti con deterioramento mentale]. Rivista di neuropsichiatria e Science Affini 1987;33(4):301‐312. [Google Scholar]
Vecchi 1991 {published data only}
- Neri M, Cortelloni C, Vreese L. Methodology of a clinical controlled study of acetyl‐l‐carnitine [Metodologia di uno studio clinico controllato con L‐acetilcarnitina]. Clinica Terapeutica 1990;132(6 Supplement):457‐68. [PubMed] [Google Scholar]
- Vecchi GP, Chiari G, Cipolli C, Cortelloni C, Vreese L, Neri M. Acetyl‐l‐carnitine treatment of mental impairment in the elderly: Evidence from a multicentre study. Archives of Gerontology and Geriatrics 1991;suppl. 2:159‐68. [Google Scholar]
- Vecchi GP, Neri M, Cortelloni C, Vreese L, Sirotti C, Cipolli C. Methodology of a controlled clinical study for Cerebral Aging. International Journal of Clinical Pharmacological Research 1990;10:145‐52. [PubMed] [Google Scholar]
References to ongoing studies
Calvani 1998 {published data only}
- Calvani M, Iannuccelli M, Lucca U. Acetyl‐L‐Carnitine in the treatment of Alzheimer's Disease patients. Conference Proceedings 6th International Conference on Alzheimer's Disease and Related Disorders; Neurobiology of Aging; 18‐23 July, 1998; Amsterdam, the Netherlands. 1998:S179.
Additional references
Ames 2004
- Ames BN, Liu J. Delaying the Mitochondrial Decay of Aging with Acetylcarnitine. Annals of the New York Academy of Sciences 2004. [DOI] [PubMed] [Google Scholar]
Aureli 1991
- Aureli T, Capuani G, Micchelli O, Ghirardi O, Ramacci MT, Conti E. Changes in brain memebrane phospholipid composition during aging: Effect of Acetyl‐l‐carnitine. Journal of Neurochemistry 1991;57(Suppl):S115. [Google Scholar]
Bella 1990
- Bella R, Bondi R, Raffaelle R, Pennisi G. Effect of acetylcarnitine on geriatric patients suffering from dysthymic disorders. International Journal of Clinical Pharmacological Research 1990. [PubMed] [Google Scholar]
Davies 1976
- Davies P, Maloney AJR. Selective loss of central cholinergic neurons in Alzheimer's Disease. Lancet 1976;308(8000):1403. [DOI] [PubMed] [Google Scholar]
DSM III‐R
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Forloni 1994
- Forloni G, Angeretti N, Smiroldo S. Neuroprotective activity of Acetyl‐l‐carnitine: studies in vitro. Journal of Neuroscience Research 1994;37(1):92‐96. [DOI] [PubMed] [Google Scholar]
Fulgente 1990
- Fulgente T, Onofroj M, Re ML, Ferracci F, Bazzano S, Ghilardi MF. Laevo‐acetylcarnitine (Nicetile) treatment of senile depression. Clin Tr J 1990. [Google Scholar]
Ghelardini 2002
- Ghelardini C. Galeotti N. Bartolini A. Amitriptyline and clomipramine activate Gi‐protein signaling pathway in the induction of analgesia. Naunyn‐Schmiedebergs Archives of Pharmacology 2002;365(1):1‐7. [DOI] [PubMed] [Google Scholar]
Girardi 1992
- Girardi O, Caprioli A, Milano S, Guiliani A, Ramacci MT, Angelucci L. Active avoidance learning in old rats chronically treated with ALCAR. Physiol Behav 1992;633:77‐82. [DOI] [PubMed] [Google Scholar]
Imperato 1989
- Imperato A. Ramacci MT. Angelucci L. Acetyl‐L‐carnitine enhances acetylcholine release in the striatum and hippocampus of awake freely moving rats. Neuroscience Letters 1989;107(1‐3):251‐5. [DOI] [PubMed] [Google Scholar]
Jorm 1987
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatrica Scandinavica 1987;76(5):465‐479. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1994;34:939‐944. [DOI] [PubMed] [Google Scholar]
Pettegrew 2000
- Pettegrew JW. Levine J. McClure RJ. Acetyl‐L‐carnitine physical‐chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Molecular Psychiatry 2000;5(6):616‐32. [DOI] [PubMed] [Google Scholar]
Rosenthal 1992
- Rosenthal R E, William R, Bogart YE, Getson PR, Fiskum G. Prevention of postischemic neurological injury through potentiation of barin energy metabolism by acetyl‐l‐carnitine. Stroke 1992. [DOI] [PubMed] [Google Scholar]
Spagnoli 1991a
- Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, Tettamanti M, Frattura L, Tiraboschi P, Comelli M, et al. Long term Acetyl‐l‐carnitine treatment in Alzheiemr's disease. Neurology 1991;31(11):1726‐1732. [DOI] [PubMed] [Google Scholar]
Tomassini 2004
- Tomassini V. Pozzilli C. Onesti E. Pasqualetti P. Marinelli F. Pisani A. Fieschi C. Comparison of the effects of acetyl L‐carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, double‐blind, crossover trial.. Journal of the Neurological Sciences 2004. [DOI] [PubMed] [Google Scholar]
Whitehouse 1982
- Whitehouse PJ, Price DL, Strible RG, et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 1982;215:1237‐1239. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hudson 2003
- Hudson S, Tabet N. Acetyl‐l‐carnitine for dementia. Cochrane Database of Systematic Reviews 2003, Issue 2. [CD003158. DOI: 10.1002/14651858.CD003158] [DOI] [PMC free article] [PubMed] [Google Scholar]


