Figure 5.
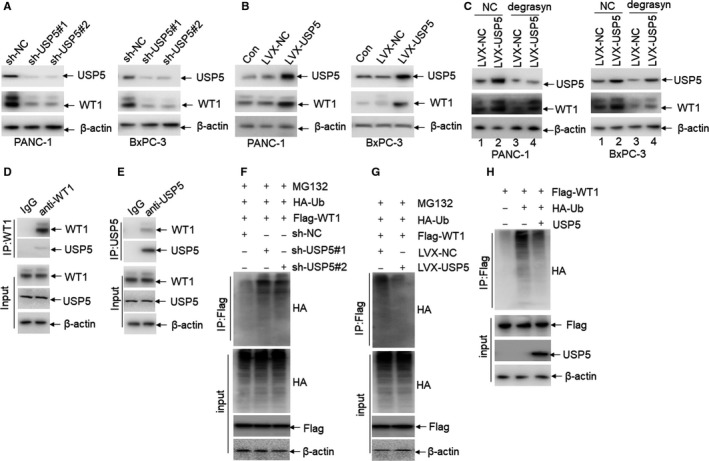
USP5 deubiquitinates WT1 protein. (A) The protein expressions of USP5 and WT1 were detected in PANC‐1 and BxPC‐3 cells, which were transduced with two specific shRNAs for USP5 (sh‐USP5#1 and sh‐USP5#2) or sh‐NC, followed by puromycin selection for seven days. (B) The protein expressions of USP5 and WT1 were detected in PANC‐1 and BxPC‐3 cells, which were transduced with LVX‐NC or LVX‐USP5 overexpressing USP5, followed by puromycin selection for seven days. (C) USP5 and WT1 expressions were detected in PANC‐1 and BxPC‐3 cells, which were transduced with LVX‐NC or LVX‐USP5 and treated with or without 1.0 μM degrasyn for 24 hours. (D) PANC‐1 lysates were immunoprecipitated by non‐specific IgG antibody and anti‐WT1 antibody, respectively. WT1 and USP5 were blotted in the immunoprecipitated lysates. (E) PANC‐1 lysates were immunoprecipitated by non‐specific IgG antibody and anti‐USP5, respectively. WT1 and USP5 were blotted in the immunoprecipitated lysates. (F) PANC‐1 cells were transduced with shRNAs for USP5 or sh‐NC together with HA‐Ub and Flag‐WT1. After treatment of MG132 (5 μM) for 4 hours, cell lysates were collected for IP assay with anti‐Flag antibody followed by Western blot with anti‐HA. (G) PANC‐1 cells were transduced with LVX‐USP5 or LVX‐NC together with HA‐Ub and Flag‐WT1, followed by MG132 (5 μM) treatment for 4 hours. Cellular extracts were prepared for IP assays with anti‐Flag followed by Western blot with anti‐HA antibody. (H) 293T cells were cotransfected with HA‐tagged ubiquitin and Flag‐WT1. Ubiquitinated‐WT1 protein was purified by anti‐Flag antibody and incubated with purified USP5 protein at 37 ℃ for 2 hours in vitro, followed by Western blot with anti‐HA antibody
