Figure 1. Physico-chemical characterization of TAP NPs.
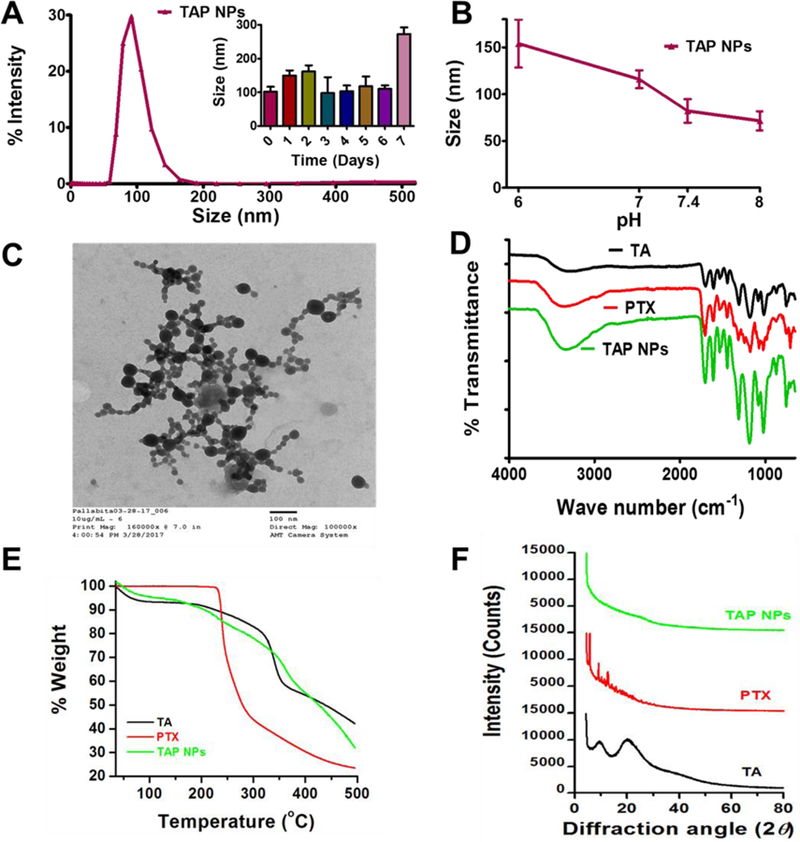
A-B) Dynamic light scattering analysis of TAP NPs: stability over 1-week and in different pH solutions. 50 µL of TAP NPs were added to 1 mL of ultrapure water or pH solutions and probe sonicated for 30 s and particle size was measured using Zetasizer (Nano ZS, Malvern Instruments, Malvern, UK) at 25 °C. A) TAP NPs exhibited particle size of 102.22±14.05 nm. Inset: representative particle size of TAP NPs stored at 25 °C for 1-week, demonstrating stability and no significant change in size. B) Stability of TAP NPs in HEPES buffer solutions (pH 6, 7, 7.4 and 8). Data presented as mean ± standard error of the mean (n = 3). C) A representative transmission electron microscopic (TEM) image of TAP NPs. UranyLess EM Stain solution was used to achieve a better contrast purpose for nanoparticles. Image was acquired by using JEOL 200EX TEM at a direct magnification of 100,000× (scale = 100 nm). D) Fourier-transform Infrared (FTIR) spectral analysis of TA, PTX and TAP NPs acquired on a Universal Attenuated Total Reflectance (UATR) accessory plate by a Spectrum 100 FTIR spectrophotometer (Waltham, MA), between 4000 and 650 cm−1 at a scanning speed of 4 cm−1 for 32 scans. TAP NPs show characteristic TA peaks at 1603 and 1700 cm−1 and PTX peaks at 3350 and 1650 cm–1, confirming the presence of functional moieties of TA and PTX in TAP NPs. E) Thermogravimetric analysis (TGA) was recorded for TA, PTX and TAP NPS from 50 to 500 °C by a Rigaku D/Max-B diffractometer (Rigaku Americas Corp, Woodlands, TX) with cobalt-alpha radiation (k = 1.5 Å). There is no significant change in thermograms of TAP NPs in comparison to TA and PTX. F) X-ray diffraction (XRD) was acquired at 2θ range of 25 – 70 oC suggesting TAP NPs has both TA and PTX present in amorphous or dissolution state.
