Fig. 1.
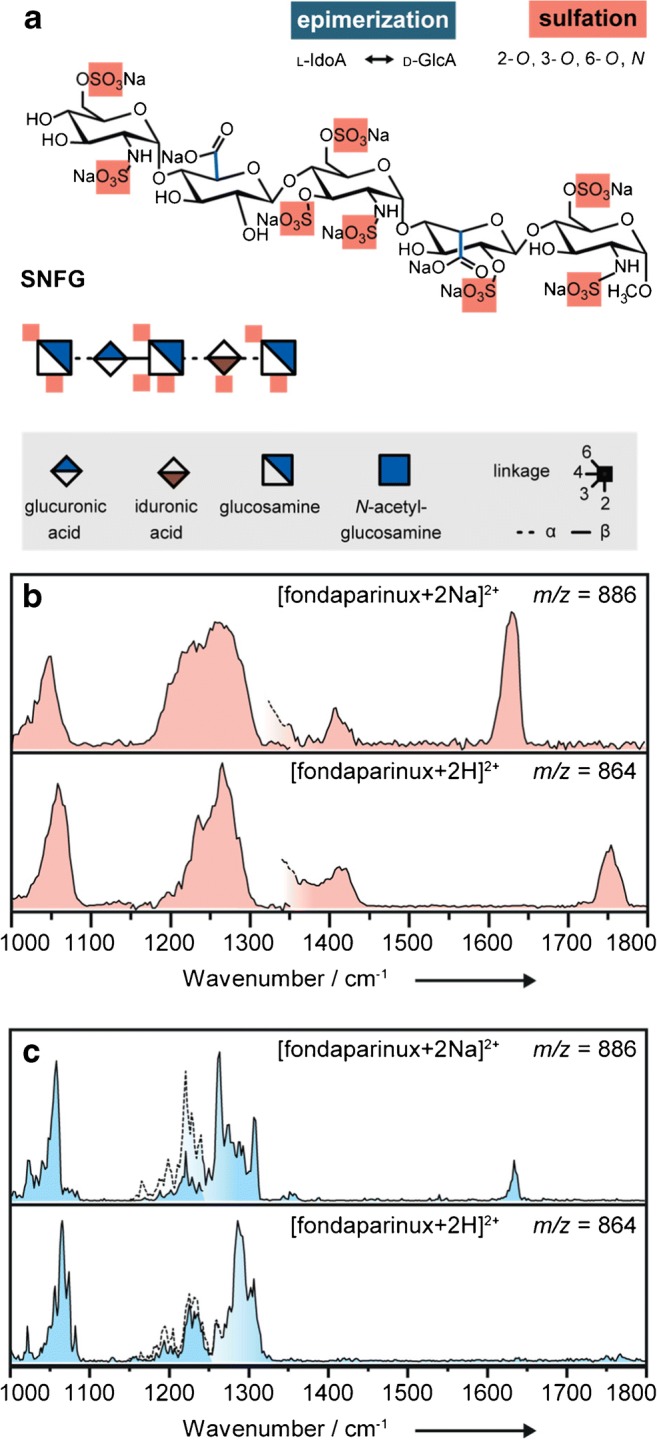
a The synthetic anticoagulant fondaparinux (as sodium salt) presented as chemical structure and in the symbol nomenclature for glycans (SNFG, legend given in grey box). Highlighted in red are the positions of the sulfate groups. b IRMPD spectroscopy of fondaparinux-sodium salt (1727 Da) investigated as adduct with two additional sodium ions [fondparinux+2Na]2+ (upper panel) and as doubly protonated species [fondaparinux+2H]2+ (lower panel).c Cryogenic IR spectroscopy in helium nanodroplets of the aforementioned ions. Dashed lines indicate an overlap of measurements using different experimental conditions (see ESM)
