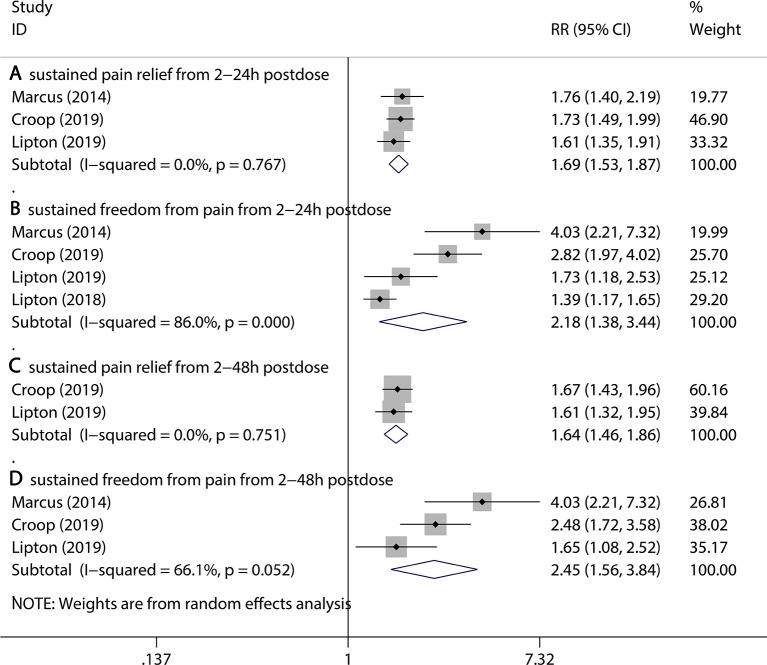
Figure 4.
The pooled RR of secondary outcomes (sustained symptoms). Notes: The black diamond indicates the estimated RR for each RCT. The gray box around each diamond indicates the estimated weight of each RCT, and the extending lines indicate the estimated 95% CI of RR for each RCT. The diamond indicates the estimated RR (95% CI) for all patients together. (A) Sustained pain relief from 2 to 24 hr post dose. (B) Sustained freedom from pain from 2 to 24 hr post dose. (C) Sustained pain relief from 2 to 48 hr post dose. (D) Sustained freedom from pain from 2 to 48 hr post dose. Weights are from a random-effects analysis. CI, confidence interval; RCT, randomized controlled trial; RR, relative risk.