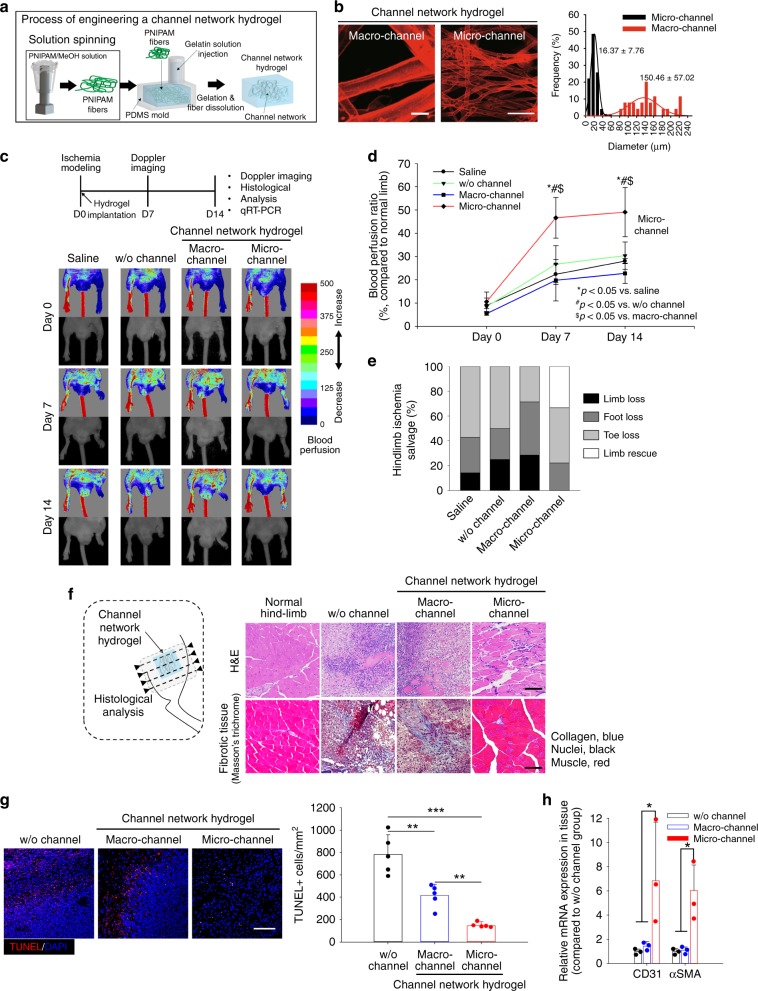
Fig. 1. Implantation of microchannel network hydrogel in mouse ischemic hindlimb tissue.
a Schematic illustration of the procedure to produce poly(N-isopropylacrylamide) (PNIPAM) fibers, then channel networks, in a hydrogel within a PDMS mold. b Confocal visualization of micro- or macrochannel networks in hydrogels with their channel diameter distribution. Channels were perfused with FluoSpheres (45 nm, red). Scale bar = 100 μm. c Laser Doppler perfusion imaging (LDPI) of supine position in a mouse model of hindlimb ischemia with d quantification of the corresponding blood perfusion ratio, compared to that of normal hindlimb at days 0, 7, and 14 post-implantation (N = 5). Statistical significances are determined using one-way ANOVA with Tukey post-hoc pairwise comparisons; *p < 0.05 versus saline; #p < 0.05 versus without (w/o) channel; and $p < 0.05 versus macrochannel group. e Fraction ratio of limb salvage in ischemic hindlimb at day 14 post surgery. f General histology (H&E: top) and fibrotic tissue staining (Masson’s trichrome: bottom) of distal hindlimb tissue site from the implant. Scale bar = 100 μm. g Cell apoptosis in hindlimb tissue at the distal site from the implant by terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assay with quantitative analysis (N = 5). Scale bars = 100 μm; **p < 0.01 and ***p < 0.005 between lined groups. h Gene expression of CD31 and alpha smooth muscle actin (αSMA) at the distal tissue site from the implant by qRT-PCR (N = 3). f–h Dots represent each animal. Data presented are mean ± SEM. Statistical significances are determined using one-way ANOVA with Tukey post-hoc pairwise comparisons; *p < 0.05 between lined groups. Source data are provided as a Source Data file.