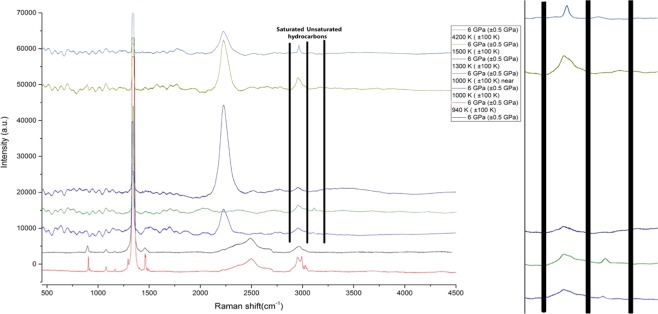
Figure 2.
Chemical transformations of propane at 6 GPa and T = 940–1600 K (±100 K). The reference peaks for C-H vibrations of saturated hydrocarbon compounds were taken from2–4,12, and for unsaturated hydrocarbons from30,32. The strong fluorescence in the region of the hydrocarbon footprint is explained by the presence of complex hydrocarbon systems having a mixed structure. The formation of ultradispersive diamonds could also affect the spectra. The propane remained stable at 940 K. The spectra of pristine propane are in good correspondence with the previous experiments that we carried out37. On the right side of the figure there is the magnified region of the C-H valence vibrations of the saturated and unsaturated hydrocarbons.