Abstract
Kashmir musk deer Moschus cupreus (KMD) are the least studied species of musk deer. We compiled genetically validated occurrence records of KMD to construct species distribution models using Maximum Entropy. We show that the distribution of KMD is limited between central Nepal on the east and north-east Afghanistan on the west and is primarily determined by precipitation of driest quarter, annual mean temperature, water vapor, and precipitation during the coldest quarter. Precipitation being the most influential determinant of distribution suggests the importance of pre-monsoon moisture for growth of the dominant vegetation, Himalayan birch Betula utilis and Himalayan fir Abies spectabilis, in KMD’s preferred forests. All four Representative Concentration Pathway Scenarios result an expansion of suitable habitat in Uttarakhand, India, west Nepal and their associated areas in China in 2050s and 2070s but a dramatic loss of suitable habitat elsewhere (Kashmir region and Pakistan-Afghanistan border). About 1/4th of the current habitat will remain as climate refugia in future. Since the existing network of protected areas will only include a tiny fraction (4%) of the climatic refugia of KMD, the fate of the species will be determined by the interplay of more urgent short-term forces of poaching and habitat degradation and long-term forces of climate change.
Subject terms: Ecological modelling, Projection and prediction
Introduction
The distribution of species around the world is not uniform; climate plays a vital role in defining species’ distributions and generating overall patterns of biodiversity in space and time. Species survive in a particular habitat mostly because they maintain an equilibrium with climate in their range1–3. Maintaining this equilibrium in the face of changing climate, however, may require a continuous shift in distribution. As the climate of the Himalaya is changing rapidly4, we expect species to adjust their distributions. Unusual patterns of precipitation and temperature have already been observed in the Himalaya and such patterns are predicted to become more severe in the future5–7. Actual and forecasted effects of climate change on biodiversity are expected to include: changes in phenology, tree line shifts, alien species invasions, declines in population and diversity, habitat alternations, and extinction8–16. Such effects could push populations of endangered species up to and beyond the verge of extinction. Hence, to maintain species diversity it is crucial to understand the impact of climate change on the distribution and habitats of endangered species, especially in the understudied areas like the Himalaya, which is already experiencing climate changes more rapidly than most of the planet is17–22.
Seven species of Musk deer Moschus spp. are endemic to the mountains of Asia and six of these are listed by the International Union for the Conservation of Nature and Natural Resources (IUCN) as endangered. Four of the endangered deers, i.e., the Kashmir musk deer Moschus cupreus, Himalayan musk deer Moschus leucogaster, Alpine musk deer Moschus chrysogaster and Black musk deer Moschus fuscus, inhabit the high Himalaya23–25. Poaching for musk and habitat fragmentation are the two major drivers of musk deer population decline26,27. Musk deer are habitat specialist, living in dense and undisturbed vegetation found above 2500 m28–32. Musk deer are a special case of convergence of challenges: they are endangered, they are habitat specialist30, and that they live in a montane system that is experiencing a rapidly changing climate33,34. Hence, it is crucially important to understand their current and future distribution if we are to keep them from going extinct. The understanding gained in examining these deer may also help us to understand the response and vulnerability of other montane species to the changing climate.
Kashmir musk deer (Moschus cupreus, hereafter KMD) is the least studied among all species of musk deer of the Himalaya because it has historically been reported only from Kashmir and associated regions of India, Pakistan and Afghanistan (Fig. 1). These areas have experienced an extended period of intense armed conflict, making research expeditions highly perilous. KMD has been described only from the Kashmir region of the western Himalaya within the altitudinal range between 2000 and 4200 m asl. Very recent studies, however, indicated that the range of the species is much wider than previously thought. Ostrowski et al.23 confirmed the presence of KMD in Nuristan, northeast Afghanistan, which is the western limit of the species. In 2019, Singh et al.35 confirmed through genetic analyses that Kashmir musk deer populations occur in the Mustang area of central Nepal, whereas the deer populations to the east of Mustang, i.e. east of Annapurna Range in the Himalaya, are Himalayan musk deer Moschus leucogaster. Hence, Mustang is the eastern limit of KMD.
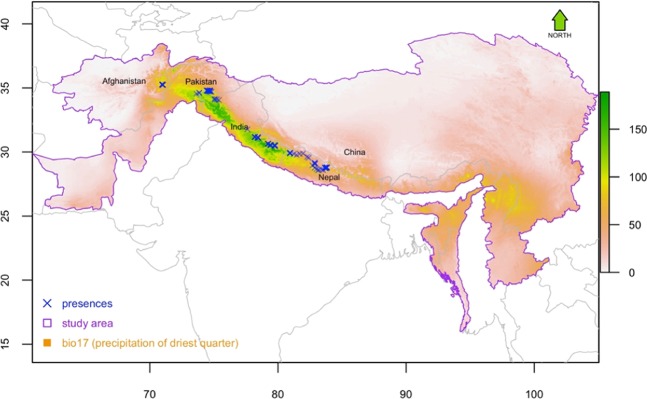
Figure 1.
Occurrences (n = 136) of Kashmir musk deer against the backdrop of precipitation of the driest quarter, which was identified by this study as the most important predictor of musk deer distribution. A total of 123 points were obtained from 10 protected areas situated between central Nepal and Afghanistan through primary data collection efforts between 2003 and 2016. These data were supplemented with 13 points from Afghanistan and Kashmir region obtained from published sources. The map was plotted using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria, https:// www.R-project.org).
Pelage color of an individual can change with the seasons and similar kinds of variation in pelage color can be found within as well as between species35–37 making pelage color an unreliable metric for species identification. The misidentification of various species resulting in a mismatch between actual and perceived range, the lack of confirmatory occurrence records, and the non-rigorous data collection scheme based on opportunistic sightings calls into question the validity of previous species distribution models. For example, two studies (Khadka et al., 2017, Khadka and James 2017) predicted the suitable habitat of Himalayan musk deer in vast stretches across the Himalaya, from east to the west and from south to the north. These results are completely unrealistic as Himalayan musk deer exist only in the eastern Himalaya, whereas Kashmir musk deer are found in the western Himalaya35,38,39. Another study Lamsal et al.40 purported to analyze the distribution of Alpine musk deer, for the entire Himalaya of Nepal, but Alpine musk deer are restricted to central China35,41. Given the misidentification of species and incorrect occurrence records in the prior studies (Khadka et al., 2017, Khadka and James 2017, Lamsal et al., 2018), it is apparent that the distribution of musk deer in the Himalaya needs to be reexamined.
In contrast to these prior studies, we did a number of things to ensure our occurrence records and habitat analyses produced high quality data. First, we used recent genetic analysis35,38,39 to confirm the populations of Kashmir musk deer and Himalayan musk deer in southern parts of the greater Himalaya. Second, we obtained distributional data of KMD by systematically surveying five protected areas in Nepal, three in India, and one in Pakistan between 2003 and 2017. Third, we searched for the locations of musk deer latrines which are set up only in high quality habitat. The deer uses latrine sites regularly throughout the year for defecation, for establishing communications with conspecifics, and to maintain territory35,42. Based on their unique location and repeated use, we suggest that latrine sites represent high quality habitat sites for musk deer, which is in contrast with opportunistic sightings (which can capture low quality habitat in the data) and unverified locations (when analysis is based on occurrence records maintained by public domain like GBIF).
In this study, we modelled the distribution of the least studied endangered Kashmir musk deer with the following objectives: (1) understand the current and expected future distribution of the species under different climatic scenarios, (2) identify potential climatic refugia for the species, and (3) determine the adequacy of the existing network of protected areas for protecting this species in future.
Results
Important predictors of species distribution
Out of 22 selected covariates, we selected for species distribution modeling, four were found to have disproportionately high influence in determining the distribution of the KMD (Fig. 2). These include precipitation of the driest quarter “bio17” (relative influence = 65%) and annual mean temperature “bio1” (relative influence = 18%), as the two top most predictors. Water vapor “vapr” and precipitation during coldest quarter “bio19” played a minor but still important role in species distribution. Collectively, the predictors possessed relative influence equal to 93% in model performance.
Figure 2.
The relative influence of the predictors in Kashmir musk deer distribution; predictors are listed on y-axis. We started our model construction process with 26 potential predictors (Table 1). However, four of these covariates (land cover, aspect, altitude and distance to the nearest water source) were not available for future climate scenarios at our desired spatial resolution or required enormous computational resources. These covariates were some of the least important predictors (relative influence <3), and therefore were dropped from further analysis. The remaining 22 predictors (Table 1) were used to develop the model.
Projected distribution under current climatic conditions
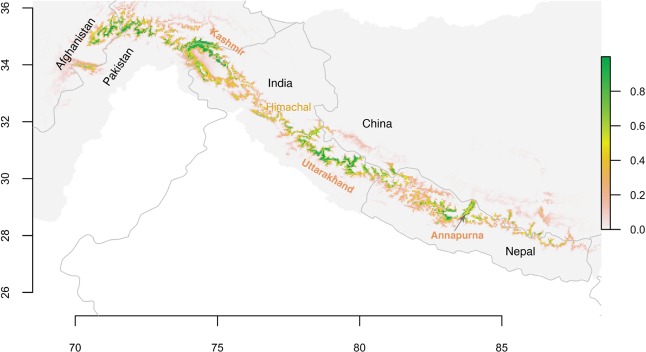
The prediction of the species distribution models yielded probability of occurrence or habitat suitability (Fig. 3). KMD is predicted to inhabit a belt of high Himalaya that stretches from central Nepal to north-west of India, reaching Afghanistan through the Kashmir Region of India and Pakistan. However, the suitable habitats do not occur in a continuum throughout the high Himalaya. Suitable habitats are located west of Annapurna Himalaya range including Mustang (hereafter Annapurna region), west of Annapurna region (hereafter, west Nepal), the northern part of Uttarakhand state of India (hereafter, Uttarakhand), west of Uttarakhand in Himachal Pradesh (hereafter Himachal), the Kashmir region of India and Pakistan (hereafter, Kashmir region), and along and close to the northeast border between Pakistan and Afghanistan (hereafter, Pak-Afghan border). Additionally, small patch of suitable habitat observed in Lubu area in China nearby north west border between Nepal and China. Few patches of suitable habitat occur in the the eastern half of Nepal and their adjacent area in Tibet as this area supports the closely related Himalayan musk deer.
Figure 3.
Habitat suitability is displayed as a continuous quantity between zero and one. The probability surface was generated by species distribution models built with Maximum Entropy (MaxEnt) Models using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria, https:// www.R-project.org).
When the continuous probability surface was classified into categories, we observed that highly suitable (probability >0.7) and suitable (probability 0.5–0.7) habitats are distributed in patches throughout the Himalaya from central Nepal to north east Afghanistan (Supplementary Fig. S1). The continuous patches of high quality habitat are located in Pak-Afghan border, Kashmir region, Uttarakhand and in Annapurna region. In other regions (Himachal, west Nepal, west of Kashmir region) suitable habitats are presented in different fragments. The areas where suitable and highly suitable habitats are presented also include marginally suitable habitat (0.2–0.5) west of Annapurna to Pak-Afghan border whereas high proportion of marginally suitable habitat are observed east of Annapurna where closely related Himalayan musk deer are present.
Projected distribution of KMD under predicted future climate scenarios
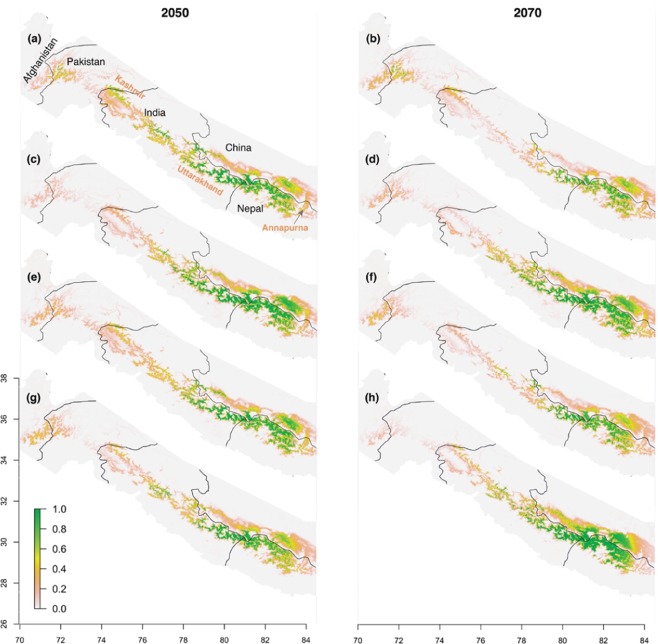
When predicted distribution under future climate change scenarios are examined collectively, northwards shifting and disappearance of habitat were observed. In west Nepal and Uttarakhand, the habitat shifts northward, resulting a continuous distribution of highly suitable habitats in north part of both areas (west Nepal and Uttarakhand) and their adjacent areas in Tibet in future in all four RCP scenarios in 2050s and 2070s. However, the extent of suitable habitat will differ in four scenarios. In 2050s (RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5), most of the suitable habitat will be available in west Nepal and Uttarakhand as well as their adjacent area in Tibet along the border between three countries (Nepal, India and China). In RCP 2.6 scenario in 2050, some patches of suitable habitat will appear in Himachal between Kashmir region and Uttarakhand in future whereas most of the habitat will disappear from Kashmir region and Pak-Afghan border. In RCP 4.5, RCP 6.0 and RCP 8.5 scenarios, by 2050, KMD’s suitable habitat will disappear from Kashmir region, Pak-Afghan border and Annapurna region. In all four scenarios during 2070s, the suitable habitat from Pak-Afghan border, Kashmir region and Himachal region will disappear. In the rest of Himalaya to the west of Uttarakhand (the Indian Himalaya, all Himalaya of Pakistan and Afghanistan Himalaya), currently observed suitable and highly suitable habitats will be entirely lost in future (2050s and 2070s) in three scenarios (RCP 45, RCP 60 and RCP 85). In the Pak-Afghan border, Pakistan, Kashmir region, and central Nepal regions only marginally suitable are predicted to be present in future during 2050s and 2070s (Fig. 4).
Figure 4.
Estimated habitat suitability in 2050 and 2070 under various climate change scenarios. The prediction was made for the entire Himalaya (see Fig. 1), but we only show the part that includes all sites with suitable habitat. (a) RCP 2.6 climate scenario in 2050s. (b) RCP 2.6 climate scenario in 2070s (c) RCP 4.5 in 2050s0s. (d) RCP 4.5 in 2070s. (e) RCP 6.0 in 2050s. (f) RCP 6.0 in 2070s. (g) RCP 8.5 in 2050s. (h) RCP 8.5 in 2070s. All maps were plotted using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org).
Compared to the other RCP scenarios (RCP 2.6, RCP 6.0 and RCP 8.5), by 2050 the habitat for KMD under the RCP 4.5 will expand more and the habitat of this deer is expected to be distributed densely within west Nepal, Uttarakhand and their associated areas in Tibet (Fig. 4). By 2070, habitat in RCP 4.5 and RCP 8.5 scenarios will depict similar pattern (Fig. 4). However, the habitat of KMD will be expanded in Uttarakhand, west Nepal and their associated areas in Tibet in RCP 8.5 scenarios (Fig. 4). The habitat in RCP 2.6 in 2070s will be found sparsely in east of Uttarakhand, west of west Nepal and their adjacent area in Tibet compare to other three scenarios. Habitat of KMD in three scenarios (RCP 26, RCP4.5 and RCP 6.0) will decrease in 2070s compare to 2050s in Uttarakhand, west Nepal and their associated areas in Tibet. However, habitat of KMD in RCP 8.5 will increase in 2070s compare to 2050s (Fig. 4).
In future, HSH (Highly Suitable Habitat) for KMD will dominate over SH (Suitable Habitat) and MSH (Marginally Suitable Habitat) in Uttarakhand and west Nepal in all four climatic scenarios. In Tibet, HSH dominate MSH and SH in three cases i.e. RCP 4.5 and RCP 6.0 in 2050s, RCP 4.5 and RCP 8.5 in 2070s whereas SH and MSH dominate HSH in five cases i.e. RCP 2.6, RCP 6.0 and RCP 8.5 in 2050, RCP 2.6, RCP 4.5, RCP 6.0. Some patches of SH will remain in Pak-Afghan border, Kashmir region and Himachal area in RCP 2.6 scenario in 2050s. In other scenarios, only MSH will exist in Pak-Afghan, Kashmir and Annapurna regions where some patches of HSH and HS will be available in Himachal areas except in RCP 2.6 in 2070s (Supplementary Fig. S2).
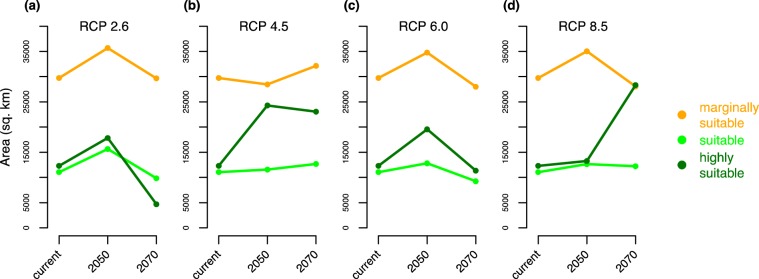
When the entire Himalaya was examined collectively, we observed that the HSH and SH will increase in all scenarios (RCP 2.6, RCP 6.0, RCP 4.5 and RCP 8.5) during 2050s whereas HSH and SH will increase than now in two scenarios (RCP 4.5 and RCP 8.5) during 2070s and decrease in remaining scenarios (RCP 2.6 and RCP 8.5) (Fig. 5). Except in 8.5 scenario in 2070s, HSH and SH will diminish than 2050s. MSH will maintain similar pattern between now and 2070s in all scenarios whereas MSH will increase in three scenarios (RCP 2.6, RCP 6.0 and RCP 8.5) in 2050s. Area of MSH will decrease in 2070s than 2050s in all scenarios except RCP 4.5. More habitat is expected to be available for KMD in Uttarakhand, west Nepal and their associated areas in Tibet in future. Both HSH and SH will dominate Uttarakhand, west Nepal and Tibet in 2050s and 2070s.
Figure 5.
Change in area of suitable habitats in future. The total area of all three types of suitable habitats was computed for all climate scenarios: RCP 2.6 (a), RCP 4.5 (b), RCP 6.0 (c) and RCP 8.5 (d). The area each grid cell was calculated separately from a raster in the Geographic Coordinate System accounting for Earth’s curvature, rather than in a flat Cartesian coordinate system.
Protected areas and refugia
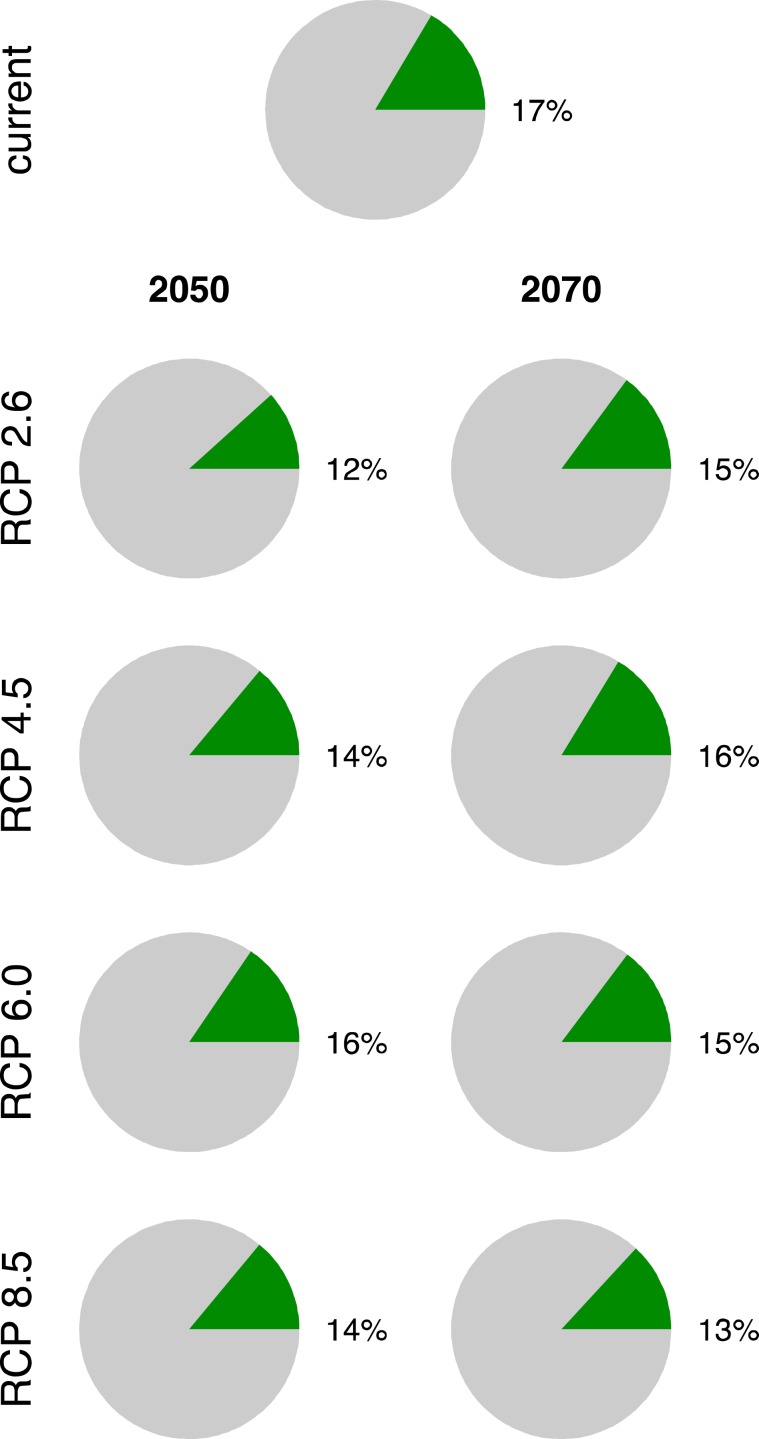
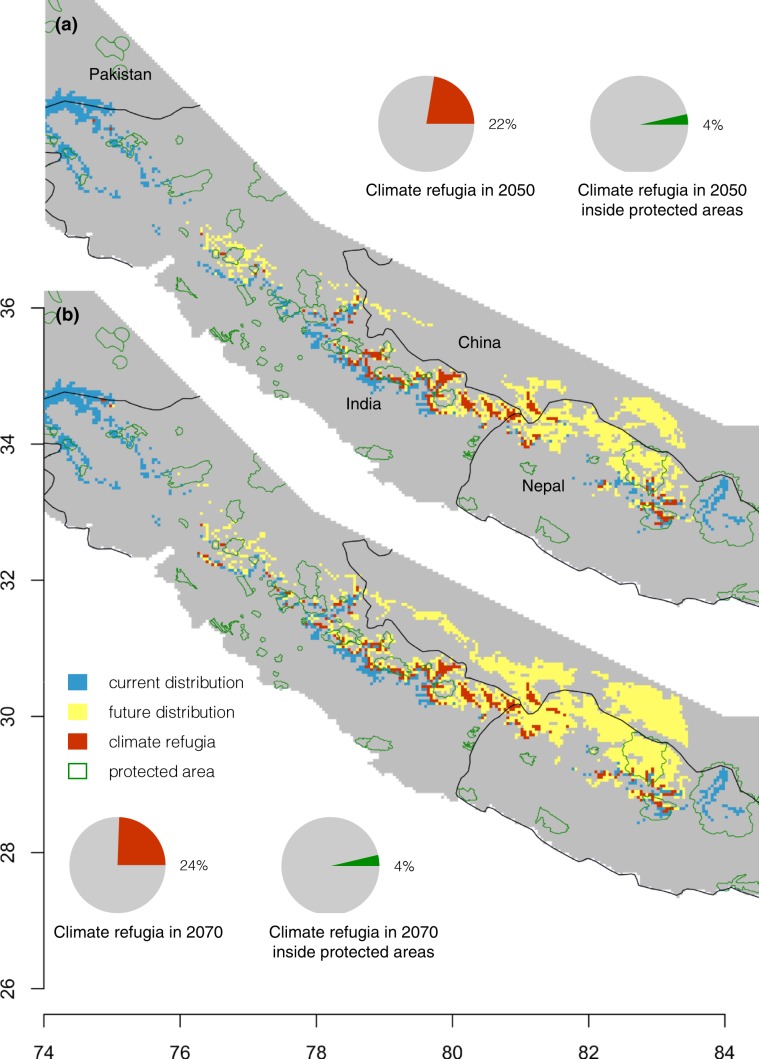
A small fraction of KMDs range now or likely in the future (Fig. 6) are being protected by the currently designated network of protected areas (green shade in Fig. 7). Currently, only 17% of range inside the protected areas contains habitat with 0.5 or greater suitability. Many of the protected areas are located north of KMD’s currently suitable habitat. As suitable habitats shift northward, more of the future range will fall outside currently protected areas (12–16%). If a site provides current and predicted future suitability, it carries higher conservation value because it could serve the purpose of a climate refugia potentially allowing that local population of KMD from needing to migrate to survive. By overlapping predicted future species range maps with the current ranges, we show that 22% and 24% of current species range will serve as climate refugia in 2050 and 2070, respectively (Fig. 7). Unfortunately, only 4% of these potential refugia are located inside present protected areas.
Figure 6.
Fraction of the projected species range that is inside currently designated protected areas. The species range is the totality of grid cells that have the probability of at least 50% suitable, and highly suitable sites. This fraction was computed as the area of the range inside a protected areas divided by the total range area. The Earth’s curvature was accounted for determining the area with the function raster:area() in R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org).
Figure 7.
Climate refugia and protected areas. Climate refugia are assumed to occur where the current species range overlaps with the predicted future range. Spatial distributions of climate refugia for 2050s (a) and for 2070s (b) under RCP 8.5 are shown. Next to each of these maps are two pie charts that display what fraction of the species current range remains as a potential climate refugia (red pie) and the fraction of that climate refugia that is located inside protected areas (green pie). All maps were plotted using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org).
Discussion
Distribution of KMD: A game of moistures
The growth limiting factor for the species of trees in KMD (Kashmir Musk Deer) habitat is the moisture available during the pre-monsoon season43–47. In the southern Himalaya, moisture comes from precipitation arising from the Indian Ocean mainly during summer, making high rainfall in the eastern Himalaya leaving the western Himalaya drier than eastern Himalaya. During winter, the westerly winds from the Mediterranean sea brings more precipitation in the form of snow to the western Himalaya, but less to the eastern Himalaya48. The massive mountain range acts as a barrier to air flow from south to north, resulting in a dry and treeless Tibet49. Most of the coniferous trees respond negatively with temperature when temperature increase above 6.6 °C and positively with precipitation during pre-monsoon50–52. Spruce Picea smithiana, fir Abies pindrow, yew Taxus wallichiana, bluepine Pinus wallichiana, Himalayan birch Betula utilis and rhododendron Rhododendron campanulatum etc. are commonly recorded tree in the habitat of KMD. Growth of these trees are limited by moisture in pre-monsoon43–45,53. Our finding also suggests that Himalayan trees are more responsive to changes in the regime of precipitation than temperature. The strong influence of precipitation to KMD distribution likely results from the sensitivity of forests supporting KMD sites to adequate rainfall. The distributional boundaries we predicted are echoed by recent studies: Singh et al.35 indicated Mustang, central Nepal as the eastern boundary and Ostrowski et al.23 indicated Nuristan, Afghanistan as the western boundary. However, the distributional range of KMD as depicted by IUCN is markedly different from our data-driven modeling studies IUCN32,35.
Future distribution of KMD
West Nepal, Uttarakhand, Kashmir region, and Pak-Afghan border will support highly suitable and suitable habitat. Most of the current habitat of KMD will disappear in the 2050s or the 2070s in all climatic scenarios (RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5) except Uttarakhand and west Nepal and their adjacent areas in Tibet. However, few patches of suitable habitat will remain in Himachal and close to Pak-Afghan border in 2050s in RCP 2.6 scenario. Mean temperature is predicted to rise by 1–2 °C in 2050s and by 1–3 °C in 2070s in four RCP scenarios54. Although temperature will keep rising, precipitation is predicted to behave differently depending on the amount of moisture in the air, where it comes from, and the convergence of air currents55,56. In the range of KMD, sites that are unsuitable today to the north of Uttarakhand and western Nepal will receive more pre-monsoon moisture in future (see Supplementary Note). This explains why those currently uninhabited areas show a suitable sites in future climates (Fig. 4).
Himalayas in Uttarakhand and west Nepal are not higher as Himalayas in Annapurna region, Pakistan and Kashmir region. Mountains above 8000 m from mean sea level are located either in Pakistan or Annapurna region and east of it. Not surprisingly this huge area and its diverse topography manifests in a very complex climate system that is not well understood57. This distinct dynamism of temperature and precipitation due to geographical differences between the western regions (Kashmir and Pak-Afghan) and the eastern region (Uttarakhand and west Nepal) are enough to justify the reason behind loss of habitat of KMD west of Uttarakhand (Kashmir region, Afghanistan and Pakistan) in future (2050s and 2070s).
Contrary to our finding, Khadka et al., 2017 found temperature play a greater role in distribution of musk deer. Therefore, Khadka et al.58 predicted (i) that vast stretches of currently suitable habitat of Himalayan musk deer exist at very high elevation including above tree line and snowline although we know that the species needs a forest to survive, and (2) that this suitable habitat expands dramatically in future to higher elevation areas without any contraction. The unrealistic future projection of musk deer by Khadka et al., 2017 has another layer of complexity: plants do not respond to newly available suitable climate above treeline and snowline because soil may not have formed in enough quantity to support the plant growth in such large area59,60. Collectively, these lines of arguments and evidences support our finding that current species range of KMD will respond to future climate in a complex fashion, which is different from a species that is primarily controlled by temperature. We show that all kinds of habitats in Kashmir region, Himachal and Pak-Afghan border will be the ones showing the first sign of climate change; they will decline in the 2050s and 2070s.
There are a few reasons why prior modeling studies (e.g., Khadka et al. 2017, Khadka and James 2017, Lamsal et al. 2018) resulted in unrealistic projection of species distribution, and they relate to the way they identified species. Pelage color is not a reliable metric for identifying species of musk deer. Pelage color may vary within a species and it changes with season. Different species can look alike as well as individuals of the same might look different35. Fecal pellets are not uniquely identified at a species level. Occurrences based on opportunistic sightings and anecdotal information has the potential to hamper the model as such occurrences have a higher chance of being incorrect or falling in lower quality habitat than our systematic survey of latrines. Khadka et al., 2017 and Khadka and James 2017 concluded that temperature (relative importance = 74%) is the most important variable influencing Himalayan musk deer distribution. In water scarce environments, a small difference in moisture availability can make a difference in habitat quality. Such ecological expectations can also be inferred from the fact that forest cover is an important factor to KMD given their timid nature30 and vegetation growth is largely influenced by moisture in dry environments60–64. These deer occur sparsely if there is inadequate dense vegetation cover (>40% crown cover) to hide within during the day30.
Protected, non-protected habitats and climate refugia
We observed that only 17% of the current suitable range (projected probability >0.5) falls inside protected areas (PAs). In the future, this will decrease to 12–16%. The range are in low fraction and other researchers have reported similarly low fraction of range protected inside conservation area for other species of Himalayan range such as 19% for snow leopard61. Large portions of the suitable habitat of musk deer are located outside PAs where anthropogenic pressure are highly threatening biodiversity65,66. Only 4% of habitat in 2050s and 2% of habitat in 2070s will act as climate refugia inside PAs. However, 25% of current suitable range in 2050s and 16% in 2070s will act as climate refugia in entire range of KMD.
Climate refugia conservation; next generation conservation
Musk deer evolved 4 million years ago in Tibetan plateau67 and survived until now despite various climate changes during the Quaternary period as well as after remarkable geological process in Asia including upliftment of Himalaya68,69. Climatic refugia are a plausible explanation for its survival through different climate changes, allowing the species later to disperse in the mountains of Asia when situation slowly normalized. Another plausible explanation for the survival relates to the ability of a species to cope with the changing environment and its interaction with other species. Unfortunately, the velocity of the climate change is faster than the response of the species is70. Therefore, the role of climate refugia is always crucial. The extinction of megafauna, such as woolly mammoth Mammuthus primigenius and Giant deer Megaloceros giganteus during the late Quaternary period was mainly because they could not find such refugia to survive through the glacial-interglacial cycle71. In the case of KMD, about quarter of the current range can serve as climate refugia in future (2050s and 2070s). These refugia are situated in Uttarakhand, west Nepal and west of Annapurna region.
Material and Methods
Study area: The greater himalaya
The greater Himalaya are often called the roof of the world and they extend from northern Pakistan to north east India throughout Nepal, Bhutan and the southern part of Tibet. Western disturbances from the west arise from the Mediterranean Sea and dominate the climate of western Himalaya during winter. During summer, the dominant weather pattern is driven by moisture arising from the Indian Ocean48,72,73. In Uttarakhand and west Nepal, the climate is different than Kashmir and Pak-Afghan as it is influenced by both south west monsoon and westerly storms74. The different weather phenomena in western and eastern Himalaya has resulted in a drier west and wetter east75. Hence, species composition are different in both regions of Himalaya. Western Himalaya temperate forests exhibits both broadleaf and conifer forest where spruce Picea smithiana, fir Abies pindrow, yew Taxus wallichiana, bluepine Pinus wallichiana and rhododendron Rhododendron campanulatum are common species. In eastern Himalaya, eastern Himalayan broadleaf forests, eastern Himalaya subalpine conifer foersts, northern trinagle temperate forests and northeastern Himalayan subalpine conifer forests form temperate forest76–78. Western tragopan Trogopan melanocephalus, cheer pheasant Catreus wallichii, Kashmir musk deer M.cupreus, Markhor Capra falconeri etc. are only found in western Himalaya whereas Alpine musk deer M. chrysogaster, Himalayan musk deer M. leucogaster, black musk deer M. fuscus, red panda Ailurus fulgen, Bhutan Takin Budorcas taxicolar whitei etc. are recorded only in eastern Himalaya.
Presence records of musk deer
Following the genetic study of the samples collected in the Nuristan region of Afghanistan and the Mustang region of Annapurna Himalaya range in Nepal (Singh et al., 2019), Nuristan is confirmed as the western limit and Mustang as as the eastern limit of the KMD range. We determined the geographic coordinates of latrines between these two distribution limits of KMD in South Asia. We documented 136 occurrence records of KMD, 123 of them being in our primary collection area within five protected areas in Nepal (Annapurna Conservation Area, Dhorpatan Hunting Reserve, Shey Phoksundo National Park; Rara National Park, Api Nampa Conservation area), Musk Deer National Park in Pakistan, Nuristan of Afghanistan, and four protected areas in India (Dachigam National Park, Kedarnath Wildlife Sanctuary, Nanda Devi Biosphere, Govinda Pashu Vihar Wildlife Sanctuary). In total, we collected 45 latrine samples in Nepal, 61 in India, 21 in Pakistan, and 9 in Afghanistan (Fig. 1).
Latrines, which serves as an indisputable evidence of deer presence, can easily be distinguished from the excreta of other animals; they have the heaps of old and fresh pellets with a musky smell42,79–81, and the pellets are much smaller and cylindrical compared to that of goat Capra aegagrus and sheep Ovis aries. Musk deer are shy, nocturnal and crepuscular forest-dweller species82. During the day, they hide in vegetation. Hence, musk deer are mostly inconspicuous to human. However, musk deer develop latrines by defecating repeatedly at a single site to maintain communication for various proposes81,83,84.
Geographical locations of latrine sites were determined first in Mustang, Nepal. In these expeditions, we performed systematic surveys for understanding habitat by laying out quadrats of size 20 m × 20 m along transects at different elevations. In the summer and autumn of 2016–2017, we visited several areas up to 4500 m in the high Himalayans of Nepal (Manang, Mustang, Kaski, Bhimthang of Annapurna region). Occurrence records from remaining four protected areas of Nepal were collected by random sightings of latrine sites and animals by the game scouts and rangers working in each protected area. For India and Pakistan, we used a retrospective study design. Coordinates of latrine sites systematically collected for Alpine musk deer from Uttarakhand, India and from Pakistan by co-author of this paper from 2003 to 2017 were considered to be KMD instead of Alpine musk deer. Data in Pakistan were collected from systematically distributed quadrats. Additionally, published literature was reviewed and presence locations of musk deer from Afghanistan and Dachigam National Park, Kashmir region, India were extracted by scanning and georeferencing previously produced range maps (Fig. 1). The data obtained from previously published sources (secondary data) comprised of less than 10% of our pool of occurrence records whereas our primary data collection effort contributed over 90% of the occurrence records.
Potential predictors
The current climate is represented by raster layers for 19 climatic variables with the resolution of 30 arc seconds (approximately 1 km) (Table 1) obtained from WorldClim version 2.085. These data were fitted to the geographic boundaries of the Hindu Kush Himalaya as defined by the International Centre for Integrated Mountain Development (ICIMOD). Based on our understanding of the distribution and ecology of KMD, we supplemented this standard set of potential covariates with additional relevant variables (Table 1). In total, 26 covariates were applied to the first set of models.
Table 1.
Potential predictors for Kashmir musk deer distribution.
| S. N. | Potential covariates | Data format | Source |
|---|---|---|---|
| 1 | wc.bio1 | Annual Mean Temperature | WorldClim 2.0 |
| 2 | wc.bio2 | Mean Diurnal Range | WorldClim 2.0 |
| 3 | wc.bio3 | Isothermally | WorldClim 2.0 |
| 4 | wc.bio4 | Temperature Seasonality | WorldClim 2.0 |
| 5 | wc.bio5 | Max Temperature of Warmest Month | WorldClim 2.0 |
| 6 | wc.bio6 | Min Temperature of Coldest Month | WorldClim 2.0 |
| 7 | wc.bio7 | Temperature Annual Range | WorldClim 2.0 |
| 8 | wc.bio8 | Mean Temperature of Wettest Quarter | WorldClim 2.0 |
| 9 | wc.bio9 | Mean Temperature of Driest Quarter | WorldClim 2.0 |
| 10 | wc.bio10 | Mean Temperature of Warmest Quarter | WorldClim 2.0 |
| 11 | wc.bio11 | Mean Temperature of Coldest Quarter | WorldClim 2.0 |
| 12 | wc.bio12 | Annual precipitation | WorldClim 2.0 |
| 13 | wc.bio13 | Precipitation of Wettest Month | WorldClim 2.0 |
| 14 | wc.bio14 | Precipitation of Dries Month | WorldClim 2.0 |
| 15 | wc.bio15 | Precipitation Seasonality | WorldClim 2.0 |
| 16 | wc.bio16 | Precipitation of Wettest Quarter | WorldClim 2.0 |
| 17 | wc.bio17 | Precipitation of Driest Quarter | WorldClim 2.0 |
| 18 | wc.bio18 | Precipitation of Warmest Quarter | WorldClim 2.0 |
| 19 | wc.bio19 | Precipitation of Coldest Quarter | WorldClim 2.0 |
| 20 | srad | Solar radiation | WorldClim 2.0 |
| 21 | vapr | Water vapor | WorldClim 2.0 |
| 22 | snowcover | Snow cover | WorldClim 2.0 |
We initially incorporated 26 covariates in species distribution model. Four of these, i.e., land cover, aspect, altitude, distance to water bodies were dropped from further analyses because of their static nature. The final model analyses included the 22 covariates listed below.
Our initial modeling exercise indicated that several of these covariates secured a very low variable importance. Some of these minimally important covariates also had other problems. Specifically, “distance to the nearest source of water” required computing distance from each of our occurrences and background points to the water bodies and identifying the nearest source. This turned out to be computationally intensive. So, we safely dropped minimally important covariates “distance to the nearest source of water”. Further, we dropped “aspect” “altitude” and “land cover” because of their static nature and they could lead to over fitting the model. We also adapted filter i.e. approach to eliminate over fitting. This approach was useful in optimizing the model coefficients such that a more stable model is trained. Specifically, we tested spatial transferability of the models by comparing various model evaluation metrics86–88. The goal here is to find a model that performs in both training and testing regions similarly. With various regularization multipliers, we found that a value of 2 minimizes the delta AIC (difference in AIC of the models in training vs testing regions). Finally, we selected 22 variables for processing our model to understand present distribution of the KMD. To forecast range patterns in the years 2050 or 2070, we used the same variables examined the present distribution of KMD in this study and applied the four climate change scenarios as used in Fifth Assessment by the International Panel on Climate Change (Representative Concentration Pathways RCP2.6, RCP4.5, RCP6, and RCP8.5). For forecasting future distribution, we used Global Circulation Model (GCM). We reviewed various literatures related to GCM. BCC-CSM1-1, CCSM4, HadGEM2-CC and MIROC 5 have been mostly used in SDM to perform future prediction. All of these models are also used in Fifth Assessment IPCC report (IPCC AR5). CMIP5 publications database reveals that CCSM4 is at top level which has been used in 440 articles, which is followed by MICROC 5 (409) and then by BCC-CCM1-1 (389). HadGEM2-CC has been used by 337 publications. We selected BCC-CSM1-1 because it is based on CCSM and itself widely used for projecting future distribution89. Therefore, using BCC-CSM1-1 provides double benefit over other GCMs. Other reasons behind applying BCC-CSM are i.) BCC-CSM1-1 is developed to issue global climate predictions and impact assessments at monthly, seasonal and inter-annual time scales, particularly over East Asiaand ii.) to do research on climate and climate change issue90. Our future predictions are related to climate change issues. We found BCC-CSM1-1 most fit in our case. All variables were resampled with the resolution of 30 arc seconds to use in model.
Maximum entropy (MaxEnt)
MaxEnt is a widely used Species Distribution Model (SDM) for predicting species distribution in the fields of ecology and conservation64,91–93. MaxEnt91 operates by computing the ratio of the probability density function of multivariate environmental space of covariates in observed locations to that of the entire study area and it is particularly suitable for modeling presence-only data. It is mathematically related to Poisson Point Process94, another model suitable for presence-only analysis. Elith and Leathwick (2009) consider MaxEnt to be a particularly efficient model and we determined it was a good choice for modeling the distribution of KMD.
An important assumption of SDM is that distributional data of a species can be collected by either randomly or systematic sampling of occurrence records95. Our occurrence data were collected systematically in Annapurna Conservation Area (Annapurna region), Api Namba Conservation Area in Nepal, Nanda Devi Biosphere, Govinda Pashu Vihar Wildlife Sanctuary but the same scheme was not used in Afghanistan, Kashmir region and far western Nepal. Therefore, it can be argued that the occurrences might have some level of sampling bias. When background points are drawn with similar bias as presences, this, in theory, dampens the impact of sampling bias in presences. As expected, Phillips et al. 2009 showed improved model performance with biased background drawing. The bias can be directly obtained from the intensity of presences in the grid cells but making sure that the grid cells without any occurrences still contribute the background points with a much smaller probability96.
We sampled background points from occupied grid cells with a bias equal to the intensity of presences. For unoccupied grid cells, we sampled background points at two probabilities: 0.01 and 0.1, and compared the predicted surface and evaluation metrics of these three schemes of background drawing (two discussed above plus one without applying any bias in the entire study area). We checked current range of the species resulted from three types of biases with the precisely known habitat of the species in reference to Himalaya range. We found that the results slightly varied among the three types of biases (AUC = 0.99 for 0.1 bias, 0.96 for 0.01 bias and 0.99 without bias). We will report results only for 0.1 bias because this secured highest AUC score. Following Guo et al., 2017, we classified the continuous probability into four categories: unsuitable (0–0.2), marginally suitable (0.2–0.5)(MH), suitable (0.5–0.7)(SH), and highly suitable (0.7–1.0)(HSH)60 to understand current and future distribution of KMD.
Analysis
All analysis and plotting were done with R 3.4.397. The main analysis of SDM was performed with the packages “dismo” and “gbm”. The following packages were used in other analyses and plotting: “ENMeval”, “raster”, “sp”, “maps”, “rgeos”, “plyr”, “matrixStats”, “scales”.
Supplementary information
Acknowledgements
This research was supported by GDAS’ Project of Science and Technology Development (2019GDASYL-0105044); the National Natural Science Foundation of China (No. 31901220, No. 31400361); The Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (2019HJ2096001006); GDAS Special Project of Science and Technology Development (2019GDASYL-0302007); GDAS Special Project of Science and Technology Development (2018GDASCX-0107, 2017GDASCX-0107). We thank National Trust for Nature Conservation, Lalitpur, Nepal for supporting us during the field work. We extend our gratitude to the CAS-TWAS Presidential Fellowship Program for providing PhD fellowship to the first author, Paras Bikram Singh. We thank to Dr. Roger Charles Helm for editing English in our manuscript. We also thank Department of National Parks and Wildlife Conservation (DNPWC) for granting permission to conduct research in the protected areas of the Himalaya and NTNC-ACAP for help during the field work. Moreover, we would like to thank to DST- SERB- Government of India for providing the financial assistance to the co-author of this article (Dr. Orus Ilyas) carry out the work. Thank is also due to the Chief Wildlife Warden of Uttarakhand and other forest staff for providing permission and giving logistic support to carry out the work in protected areas.
Author contributions
P.B.S. and K.M. were involved with the concept and design. P.B.S. collected field data from Nepal, M.N.A. and O.I. collected field data from Pakistan and India respectively. P.B.S. and K.M. conducted the analysis. P.B.S. led the writing and all authors were involved in interpretation of the results and in the writing process. All authors approved the final draft of the manuscript.
Data availability
Musk deer is endangered species and poaching for the musk is major cause of population decline of the deer. Our presence records are location of latrine sites and it was found that poachers rely on freshness of latrines site to track the deer. These sites are used repeatedly by musk deer for many years. Therefore, we can not make our presence data publicly available. However, we can provide these data upon the formal request from the researchers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58111-6.
References
- 1.Araújo MB, Pearson RG. Equilibrium of species’ distributions with climate. Ecography. 2005;28:693–695. doi: 10.1111/j.2005.0906-7590.04253.x. [DOI] [Google Scholar]
- 2.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist. 1959;93:145–159. doi: 10.1086/282070. [DOI] [Google Scholar]
- 4.Chaudhary, P. & Bawa, K. S. Local perceptions of climate change validated by scientific evidence in the Himalayas. Biology Letters, rsbl20110269 (2011). [DOI] [PMC free article] [PubMed]
- 5.Solomon, S. Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC. Vol. 4 (Cambridge University Press, 2007).
- 6.Shrestha AB, Wake CP, Dibb JE, Mayewski PA. Precipitation fluctuations in the Nepal Himalaya and its vicinity and relationship with some large scale climatological parameters. International Journal of Climatology. 2000;20:317–327. doi: 10.1002/(SICI)1097-0088(20000315)20:3<317::AID-JOC476>3.0.CO;2-G. [DOI] [Google Scholar]
- 7.Xu J, et al. The melting Himalayas: cascading effects of climate change on water, biodiversity, and livelihoods. Conservation Biology. 2009;23:520–530. doi: 10.1111/j.1523-1739.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 8.Salick J, Ghimire SK, Fang Z, Dema S, Konchar KM. Himalayan alpine vegetation, climate change and mitigation. Journal of Ethnobiology. 2014;34:276–293. doi: 10.2993/0278-0771-34.3.276. [DOI] [Google Scholar]
- 9.Aryal A, Brunton D, Raubenheimer D. Impact of climate change on human-wildlife-ecosystem interactions in the Trans-Himalaya region of Nepal. Theoretical and applied climatology. 2014;115:517–529. doi: 10.1007/s00704-013-0902-4. [DOI] [Google Scholar]
- 10.Harsch MA, Hulme PE, McGlone MS, Duncan RP. Are treelines advancing? A global meta‐analysis of treeline response to climate warming. Ecology letters. 2009;12:1040–1049. doi: 10.1111/j.1461-0248.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 11.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 12.Brown CJ, et al. Ecological and methodological drivers of species’ distribution and phenology responses to climate change. Global change biology. 2016;22:1548–1560. doi: 10.1111/gcb.13184. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharjee A, et al. The Impact of Climate Change on Biodiversity in Nepal: Current Knowledge, Lacunae, and Opportunities. Climate. 2017;5:80. doi: 10.3390/cli5040080. [DOI] [Google Scholar]
- 14.Mainali KP, et al. Projecting future expansion of invasive species: comparing and improving methodologies for species distribution modeling. Global change biology. 2015;21:4464–4480. doi: 10.1111/gcb.13038. [DOI] [PubMed] [Google Scholar]
- 15.Smeraldo S, et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: lessons from bats. Biodiversity and conservation. 2018;27:2425–2441. doi: 10.1007/s10531-018-1545-7. [DOI] [Google Scholar]
- 16.Soroye P, Ahmed N, Kerr JT. Opportunistic citizen science data transform understanding of species distributions, phenology, and diversity gradients for global change research. Global change biology. 2018;24:5281–5291. doi: 10.1111/gcb.14358. [DOI] [PubMed] [Google Scholar]
- 17.Singh SP, Singh V, Skutsch M. Rapid warming in the Himalayas: Ecosystem responses and development options. Climate and development. 2010;2:221–232. doi: 10.3763/cdev.2010.0048. [DOI] [Google Scholar]
- 18.Wang W, Xiang Y, Gao Y, Lu A, Yao T. Rapid expansion of glacial lakes caused by climate and glacier retreat in the Central Himalayas. Hydrological Processes. 2015;29:859–874. doi: 10.1002/hyp.10199. [DOI] [Google Scholar]
- 19.Rignot, E. et al. The Copenhagen diagnosis, 2009: updating the world on the latest climate science (2011).
- 20.IPCC. Global Warming of 1.5 °C. 26 (Intergovernmental Panel on Climate Change, Switzerland, 2018).
- 21.Mohammadi S, Ebrahimi E, Moghadam MS, Bosso L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecological Informatics. 2019;52:7–13. doi: 10.1016/j.ecoinf.2019.04.003. [DOI] [Google Scholar]
- 22.Moat J, Gole TW, Davis AP. Least Concern to Endangered: Applying climate change projections profoundly influences the extinction risk assessment for wild Arabica coffee. Global change biology. 2019;25:390–403. doi: 10.1111/gcb.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrowski S, Rahmani H, Ali JM, Ali R, Zahler P. Musk deer Moschus cupreus persist in the eastern forests of Afghanistan. Oryx. 2016;50:323–328. doi: 10.1017/S0030605314000611. [DOI] [Google Scholar]
- 24.Zhou Y, Meng X, Feng J, Yang Q. Review of the distribution, status and conservation of musk deer in China. Folia Zoologica. 2004;53:129–140. [Google Scholar]
- 25.Wilson, D. E. M. & Russell, A. Handbook of the Mammals of the World. Vol. 2 320–336 (Lynx edicions 2011).
- 26.Green MJ. The distribution, status and conservation of the Himalayan musk deer Moschus chrysogaster. Biological conservation. 1986;35:347–375. doi: 10.1016/0006-3207(86)90094-7. [DOI] [Google Scholar]
- 27.Homes, V. No Licence to Kill: The Population and Harvest of Musk Deer and Trade in Musk in the Russian Federation and Mongolia. (Traffic Europe, Brussels, Belgium, 2004).
- 28.Shrestha BB, Moe SR. Plant diversity and composition associated with Himalayan musk deer latrine sites. Zoology and Ecology. 2015;25:295–304. doi: 10.1080/21658005.2015.1069498. [DOI] [Google Scholar]
- 29.Shrestha, B. B. & Meng, X. Spring habitat preference, association and threats of Himalayan musk deer (Moschus leucogaster) in Gaurishankar Conservation Area, Nepal. International Journal of Conservation Science5 (2014).
- 30.Singh, P. et al. Ecological correlates of Himalayan musk deer. Ecology and Evolution, 1–15 (2018). [DOI] [PMC free article] [PubMed]
- 31.Timmins, R. J. & Duckworth, J. W. Moschus leucogaster. The IUCN Red List of Threatened Species 2015: e.T13901A61977764., http://www.iucnredlist.org/details/13901/0 (2015).
- 32.Timmins, R. J. & Duckworth, J. W. Moschus cupreus. The IUCN Red List of Threatened Species 2015: e.T13901A61977764. http://www.iucnredlist.org/details/13901/0. (2015).
- 33.Isaac, R., Shakti, S., Hardeep, M. & Isaac, M. In Селевые потоки: катастрофы, риск, прогноз, защита. 115–122.
- 34.Salick J, Fang Z, Hart R. Rapid changes in eastern Himalayan alpine flora with climate change. American journal of botany. 2019;106:520–530. doi: 10.1002/ajb2.1263. [DOI] [PubMed] [Google Scholar]
- 35.Singh, P. B., Khatiwada, J. R., Saud, P. & Zhigang, J. mtDNA analysis confirms the endangered kashmir musk deer extends its range to Nepal. Scientific reports5 (2019). [DOI] [PMC free article] [PubMed]
- 36.Groves, C. P. & Grubb, P. Ungulate taxonomy. (The John Hopkins University Press, 2011).
- 37.Liu Z, Groves C. Taxonomic diversity and colour diversity: rethinking the taxonomy of recent musk deer (Moschus, Moschidae, Ruminantia) Gazella. 2014;41:73–97. [Google Scholar]
- 38.Guo, K., Li, F., Zhang, Q. & Chen, S. Complete mitochondrial genome of the Himalayan Musk Deer, Moschus leucogaster, with phylogenetic implication. Conservation Genetics Resources, 1–4 (2018).
- 39.Shrestha B, Khatiwada J, Thanet D. mtDNA confirms the presence of Moschus leucogaster (Ruminantia, Moschidae) in Gaurishankar Conservation Area, Nepal. Miscel· lània Zoològica. 2018;17:209–218. [Google Scholar]
- 40.Lamsal P, Kumar L, Aryal A, Atreya K. Future climate and habitat distribution of Himalayan Musk Deer (Moschus chrysogaster) Ecological Informatics. 2018;44:101–108. doi: 10.1016/j.ecoinf.2018.02.004. [DOI] [Google Scholar]
- 41.Harris, R. The IUCN Red List of Threatened Species 2016: e.T13895A61977139., http://iucnredlist.org/ (2016).
- 42.Green MJ. Scent marking in the Himalayan musk deer (Moschus chrysogaster) Journal of Zoology. 1987;1:721–737. doi: 10.1111/j.1096-3642.1987.tb00752.x. [DOI] [Google Scholar]
- 43.Dawadi B, Liang E, Tian L, Devkota LP, Yao T. Pre-monsoon precipitation signal in tree rings of timberline Betula utilis in the central Himalayas. Quaternary International. 2013;283:72–77. doi: 10.1016/j.quaint.2012.05.039. [DOI] [Google Scholar]
- 44.Gaire NP, et al. Tree-ring based spring precipitation reconstruction in western Nepal Himalaya since AD 1840. Dendrochronologia. 2017;42:21–30. doi: 10.1016/j.dendro.2016.12.004. [DOI] [Google Scholar]
- 45.Sohar K, Altman J, Lehečková E, Doležal J. Growth–climate relationships of Himalayan conifers along elevational and latitudinal gradients. International Journal of Climatology. 2017;37:2593–2605. doi: 10.1002/joc.4867. [DOI] [Google Scholar]
- 46.Tiwari A, Fan Z-X, Jump AS, Li S-F, Zhou Z-K. Gradual expansion of moisture sensitive Abies spectabilis forest in the Trans-Himalayan zone of central Nepal associated with climate change. Dendrochronologia. 2017;41:34–43. doi: 10.1016/j.dendro.2016.01.006. [DOI] [Google Scholar]
- 47.Bräuning A. Tree-ring studies in the Dolpo-Himalya (western Nepal). Tree Rings in. Archaeology, Climatology and Ecology. 2004;2:8–12. [Google Scholar]
- 48.Sigdel M, Ikeda M. Seasonal contrast in precipitation mechanisms over Nepal deduced from relationship with the large-scale climate patterns. Nepal Journal of Science and Technology. 2013;13:115–123. doi: 10.3126/njst.v13i1.7450. [DOI] [Google Scholar]
- 49.Shekhar M, et al. Winter precipitation climatology over Western Himalaya: Altitude and Range wise study. J. Ind. Geophys. Union (March 2017) 2017;21:148–152. [Google Scholar]
- 50.Borgaonkar H, Sikder A, Ram S. High altitude forest sensitivity to the recent warming: a tree-ring analysis of conifers from Western Himalaya, India. Quaternary International. 2011;236:158–166. doi: 10.1016/j.quaint.2010.01.016. [DOI] [Google Scholar]
- 51.Yadav, R. R., Park, W. K., Singh, J. & Dubey, B. Do the western Himalayas defy global warming? Geophysical Research Letters31 (2004).
- 52.Singh J, Yadav RR, Wilmking M. A 694-year tree-ring based rainfall reconstruction from Himachal Pradesh, India. Climate Dynamics. 2009;33:1149. doi: 10.1007/s00382-009-0528-5. [DOI] [Google Scholar]
- 53.Panthi S, Bräuning A, Zhou Z-K, Fan Z-X. Tree rings reveal recent intensified spring drought in the central Himalaya, Nepal. Global and Planetary Change. 2017;157:26–34. doi: 10.1016/j.gloplacha.2017.08.012. [DOI] [Google Scholar]
- 54.IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 150 (IPCC, Geneva, Switzerland, 2014).
- 55.Allen MR, Ingram WJ. Constraints on future changes in climate and the hydrologic cycle. Nature. 2002;419:224. doi: 10.1038/nature01092. [DOI] [PubMed] [Google Scholar]
- 56.Trenberth KE. Changes in precipitation with climate change. Climate Research. 2011;47:123–138. doi: 10.3354/cr00953. [DOI] [Google Scholar]
- 57.Duan, K., Yao, T. & Thompson, L. G. Response of monsoon precipitation in the Himalayas to global warming. Journal of Geophysical Research: Atmospheres111 (2006).
- 58.Khadka KK, Kannan R, Ilyas O, Abbas F-I, James DA. Where are they? Where will they be? In pursuit of current and future whereabouts of endangered Himalayan musk deer. Mammalian Biology-Zeitschrift für Säugetierkunde. 2017;85:30–36. doi: 10.1016/j.mambio.2017.02.007. [DOI] [Google Scholar]
- 59.Telwala Y, Brook BW, Manish K, Pandit MK. Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS One. 2013;8:e57103. doi: 10.1371/journal.pone.0057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Y, et al. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Scientific reports. 2017;7:46221. doi: 10.1038/srep46221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, et al. Climate refugia of snow leopards in High Asia. Biological Conservation. 2016;203:188–196. doi: 10.1016/j.biocon.2016.09.026. [DOI] [Google Scholar]
- 62.Kandel K, et al. Rapid multi-nation distribution assessment of a charismatic conservation species using open access ensemble model GIS predictions: Red panda (Ailurus fulgens) in the Hindu-Kush Himalaya region. Biological Conservation. 2015;181:150–161. doi: 10.1016/j.biocon.2014.10.007. [DOI] [Google Scholar]
- 63.Shrestha UB, Bawa KS. Impact of climate change on potential distribution of Chinese caterpillar fungus (Ophiocordyceps sinensis) in Nepal Himalaya. PLoS one. 2014;9:e106405. doi: 10.1371/journal.pone.0106405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thapa A, et al. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecology and evolution. 2018;8:10542–10554. doi: 10.1002/ece3.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandit M, Sodhi NS, Koh LP, Bhaskar A, Brook BW. Unreported yet massive deforestation driving loss of endemic biodiversity in Indian Himalaya. Biodiversity and Conservation. 2007;16:153–163. doi: 10.1007/s10531-006-9038-5. [DOI] [Google Scholar]
- 66.Grumbine RE, Pandit MK. Threats from India’s Himalaya dams. Science. 2013;339:36–37. doi: 10.1126/science.1227211. [DOI] [PubMed] [Google Scholar]
- 67.Pan T, et al. Species delimitation in the genus Moschus (Ruminantia: Moschidae) and its high-plateau origin. PloS one. 2015;10:e0134183. doi: 10.1371/journal.pone.0134183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung S-L, et al. Diachronous uplift of the Tibetan plateau starting 40 Myr ago. Nature. 1998;394:769–773. doi: 10.1038/29511. [DOI] [Google Scholar]
- 69.Zhang D, Fengquan L, Jianmin B. Eco-environmental effects of the Qinghai-Tibet Plateau uplift during the Quaternary in China. Environmental Geology. 2000;39:1352–1358. doi: 10.1007/s002540000174. [DOI] [Google Scholar]
- 70.Loarie SR, et al. The velocity of climate change. Nature. 2009;462:1052. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 71.Cooper A, et al. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science. 2015;349:602–606. doi: 10.1126/science.aac4315. [DOI] [PubMed] [Google Scholar]
- 72.Fukada, K. The Great Himalayas. Himalayas, Harry N. Abrahams, Inc.;New York (1971).
- 73.Panthi J, et al. Spatial and temporal variability of rainfall in the Gandaki River Basin of Nepal Himalaya. Climate. 2015;3:210–226. doi: 10.3390/cli3010210. [DOI] [Google Scholar]
- 74.Bhambri R, Bolch T, Chaujar RK, Kulshreshtha SC. Glacier changes in the Garhwal Himalaya, India, from 1968 to 2006 based on remote sensing. Journal of Glaciology. 2011;57:543–556. doi: 10.3189/002214311796905604. [DOI] [Google Scholar]
- 75.Wang L, et al. Conservation priorities of forest ecosystems with evaluations of connectivity and future threats: Implications in the Eastern Himalaya of China. Biological Conservation. 2016;195:128–135. doi: 10.1016/j.biocon.2015.12.044. [DOI] [Google Scholar]
- 76.Khan SM, Page S, Ahmad H, Shaheen H, Harper D. Vegetation dynamics in the Western Himalayas, diversity indices and climate change. Sci., Tech. and Dev. 2012;31:232–243. [Google Scholar]
- 77.Chettri N, Sharma E, Zomer R. Changing paradigm and post 2010 targets: Challenges and opportunities for biodiversity conservation in the Hindu Kush Himalayas. Tropical Ecology. 2012;53:245–259. [Google Scholar]
- 78.CEPF. Ecosystem Profile, Eastern Himalayas Region. WWF-US, Asia Program (2005).
- 79.Meng X, Cody N, Gong B, Xiang L. Stable fighting strategies to maintain social ranks in captive male Alpine musk deer (Moschus sifanicus) Animal Science Journal. 2012;83:617–622. doi: 10.1111/j.1740-0929.2011.01007.x. [DOI] [PubMed] [Google Scholar]
- 80.Meng Q, Li H, Meng X. Behavior pattern as the indicator of reproductive success of Alpine musk deer. Iranian Journal of Veterinary Research. 2012;13:276–281. [Google Scholar]
- 81.Singh PB, Shrestha BB, Thapa A, Saud P, Jiang Z. Selection of latrine sites by Himalayan musk deer (Moschus leucogaster) in Neshyang Valley, Annapurna Conservation Area, Nepal. Journal of Applied Animal Research. 2018;46:920–926. doi: 10.1080/09712119.2018.1430578. [DOI] [Google Scholar]
- 82.Sheng, H. & Ohtaishi, N. Deer of China: Biology and Management. (Elsevier Science Publishers, Amsterdam. 1993).
- 83.Green, M. J. B. Aspects of the ecology of the Himalayan musk deer, University of Cambridge, Cambridge,UK (1985).
- 84.Kattel, B. Ecology of the Himalayan musk deer in Sagarmatha National Park, Nepal, Colorado State University, USA, (1993).
- 85.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 86.Di Cola V, et al. ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography. 2017;40:774–787. doi: 10.1111/ecog.02671. [DOI] [Google Scholar]
- 87.Qiao H, et al. An evaluation of transferability of ecological niche models. Ecography. 2019;42:521–534. doi: 10.1111/ecog.03986. [DOI] [Google Scholar]
- 88.Muscarella R, et al. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution. 2014;5:1198–1205. doi: 10.1111/2041-210X.12261. [DOI] [Google Scholar]
- 89.Xiaoge X, Li Z, Jie Z, Tongwen W, Yongjie F. Climate change projections over East Asia with BCC_CSM1. 1 climate model under RCP scenarios. 気象集誌. 第 2 輯. 2013;91:413–429. [Google Scholar]
- 90.Wu T, et al. An overview of BCC climate system model development and application for climate change studies. Journal of Meteorological Research. 2014;28:34–56. [Google Scholar]
- 91.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. doi: 10.1111/j.0906-7590.2008.5203.x. [DOI] [Google Scholar]
- 92.Mothes CC, Stroud JT, Clements SL, Searcy CA. Evaluating ecological niche model accuracy in predicting biotic invasions using South Florida’s exotic lizard community. Journal of biogeography. 2019;46:432–441. doi: 10.1111/jbi.13511. [DOI] [Google Scholar]
- 93.Bosso L, et al. Loss of potential bat habitat following a severe wildfire: a model-based rapid assessment. International Journal of Wildland Fire. 2018;27:756–769. doi: 10.1071/WF18072. [DOI] [Google Scholar]
- 94.Renner IW, Warton DI. Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology. Biometrics. 2013;69:274–281. doi: 10.1111/j.1541-0420.2012.01824.x. [DOI] [PubMed] [Google Scholar]
- 95.Phillips SJ, et al. Sample selection bias and presence‐only distribution models: implications for background and pseudo‐absence data. Ecological applications. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 96.Kramer‐Schadt S, et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Diversity and Distributions. 2013;19:1366–1379. doi: 10.1111/ddi.12096. [DOI] [Google Scholar]
- 97.R Core Team. R: A language and environment for statistical computing., https://www.r-project.org/ (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Musk deer is endangered species and poaching for the musk is major cause of population decline of the deer. Our presence records are location of latrine sites and it was found that poachers rely on freshness of latrines site to track the deer. These sites are used repeatedly by musk deer for many years. Therefore, we can not make our presence data publicly available. However, we can provide these data upon the formal request from the researchers.