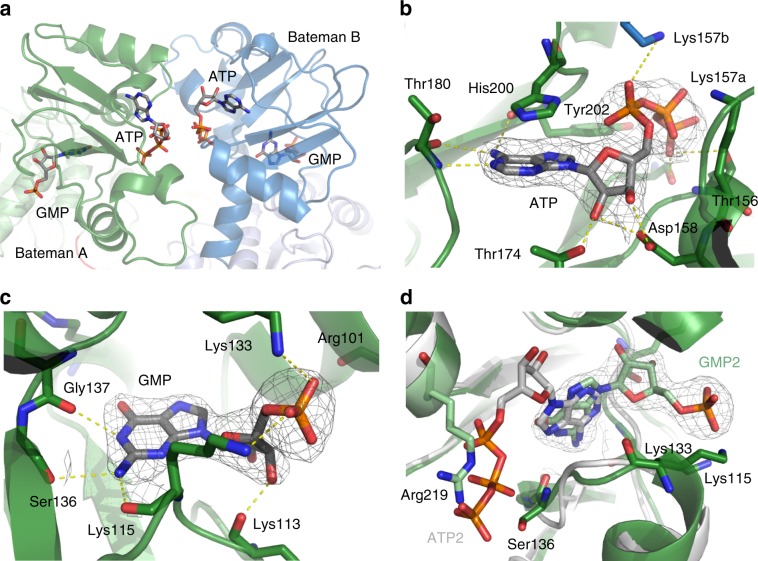
Fig. 5. Nucleotide binding to the Bateman domain of TbIMPDH.
a Electron density detected in two clefts on the surface of the Bateman domain of each monomer in the ASU was assigned to the coordination of one ATP and one GMP molecule in the canonical nucleotide binding sites. b, c Detailed view of the ATP and GMP binding. The protein is shown as green cartoons and the nucleotides as gray sticks. The side chain of Lys157’ from the neighboring monomer is colored in blue. The ATP and GMP as well as interacting residues are displayed as sticks. Yellow dashes represent hydrogen bonds. d Superposition of the second canonical binding site of TbIMPDH-ATP1/GMP2 (green) with that of AgIMPDH-ATP1/ATP2 (gray). Due to the flipped binding mode of GMP2 in TbIMPDH, the side chain of Arg219 occupies the space required for the phosphate groups of ATP2 in AgIMPDH. In b–d, the displayed 2FoFc-electron density is countered at 1.0 sigma.