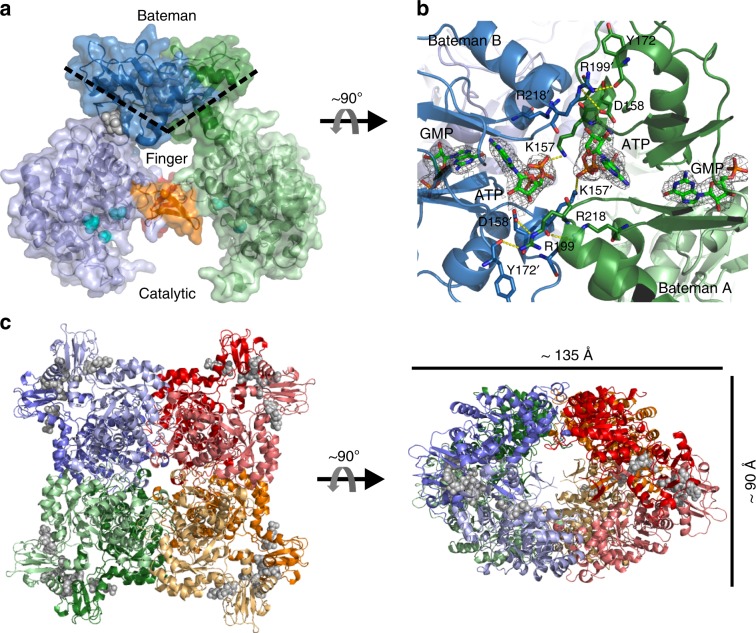
Fig. 7. Quaternary structure of TbIMPDH-ATP1/GMP2.
a Relative orientation of two TbIMPDH monomers located in the ASU, estimated by the approximate angle spanned by the two beta sheets of the CBS motifs in each Bateman domain, as indicated by the dashed line. The finger domains are highlighted (orange/red), the catalytic residues (cyan), and the bound nucleotides (gray) are shown as spheres. b Detailed view on the interface formed by the adjacent Bateman domains (green/blue, cartoon representation) within the TbIMPDH dimer. Residues involved in the dimer interaction (yellow dashes) and ATP/GMP molecules are shown as sticks. The displayed 2FoFc-electron density of ATP and GMP is countered at 1.0 sigma. c Cartoon representation of the octamer assembly of TbIMPDH monomers, as observed within the in cellulo crystals and corresponding space group, in different views rotated by 90°. Four dimers are forming an octamer around the 4-fold symmetry axis. The dimeric building blocks observed within the ASU are individually colored. Bound nucleotides are shown in a gray spheres representation.