Abstract
Background
The pharmacokinetics of temozolomide (TMZ) in patients with severe renal impairments (creatinine clearance, <36 mL/min/m2) or in hemodialysis (HD) patients has not been investigated. TMZ and its metabolic products are mainly excreted in urine, as retention of these in the body may result in increased adverse events in HD patients.
Methods
Seven HD patients with high-grade gliomas from 6 institutions were included in the study. Patient characteristics, treatment schedule, clinical course, pathological/molecular findings, and adverse events were evaluated.
Results
The histopathological diagnoses were isocitrate dehydrogenase (IDH) wild-type glioblastoma in 4 cases, not other specified (NOS) glioblastoma in 2 cases, and IDH-mutant anaplastic astrocytoma in 1 case. Five of the 7 patients completed radiotherapy (48-60 Gy) with concomitant TMZ (75 mg/m2) followed by adjuvant 5-day TMZ (150 mg/m2) every 28 days. During the entire course of treatment with TMZ, severe (Common Terminology Criteria for Adverse Events [CTCAE] ≥ Grade 3) lymphocytopenia occurred in 57%, neutropenia in 0%, and thrombocytopenia in 14% of the patients. Generally, the frequency and degree of myelosuppression do not increase in HD patients with high-grade gliomas. Two of the 7 (28.5%) patients died of infectious disease despite having no direct correlation to myelosuppression; that is similar to the death rate of 21.9% resulting from infection in HD patients in Japan.
Conclusions
Decreasing the dose of TMZ might not be required in HD patients with high-grade gliomas during concomitant radiochemotherapy and maintenance therapy. However, careful clinical and hematological observation is required to avoid critical hematotoxicity and infection.
Keywords: adverse events, hemodialysis patients, malignant glioma, temozolomide
Temozolomide (TMZ) is proven to be effective in treating malignant gliomas.1 TMZ, an imidazotetrazine derivative that is stable at acidic pH less than 5 and labile at pH greater than 7, can be administered orally and intravenously. At physiologic pH, TMZ is spontaneously hydrolyzed to its active metabolite, 5-(3-dimethyl-1-triazenyl) imidazole-4-carboxamide (MTIC), which is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC). AIC is a known intermediate in purine and nucleic acid biosynthesis and is converted to methylhydrazine, which is the active alkylating species. Cytochrome P450 enzymes play only minor roles in the metabolism of TMZ and MTIC.2 A population pharmacokinetic analysis revealed that age (range, 19-78 years) has no influence on the pharmacokinetics of TMZ.2 Another population pharmacokinetic analysis revealed that creatinine clearance (range, 36-130 mL/min/m2) had no effect on TMZ clearance after oral administration. However, the pharmacokinetics of TMZ in patients with severe renal impairments (creatinine clearance, <36 mL/min/m2) and in hemodialysis (HD) patients has not yet been investigated, and the TMZ prescription information states only that “caution should be exercised when temozolomide is administered to patients with severe renal impairment.” 2
TMZ has not been studied in HD patients. The bioavailability of TMZ on oral administration has been found to be almost 100%,3 and about 38% of the total administered TMZ radioactive dose is recovered over 7 days: 37.7% in the urine and 0.8% in feces, respectively. Radioactivity is recovered in the urine as TMZ (5.6%), AIC (12%), acid metabolite (2.3%), and unidentified polar metabolites (17%).2 Radioactive TMZ was used to investigate the excretion of TMZ from the body. Therefore, TMZ or its metabolites that could be converted into methylhydrazine might excessively remain in patients with chronic kidney disease, enhancing TMZ-associated adverse events (AEs) in those patients.4
In the present study, we report a case series of HD patients with high-grade gliomas who were treated with TMZ at multiple institutions, and we discuss whether TMZ doses should be decreased in HD patients with high-grade gliomas.
Materials and Methods
Seven HD patients who underwent radiochemotherapy with TMZ were collected from 7 institutions. Clinical data were obtained from the patients’ records, including age, sex, histopathological diagnosis, molecular information of the tumors, prescribed treatment, AEs during the concomitant and adjuvant TMZ therapy, KPS and modified Rankin scale before and after initial treatment, radiographic findings before and after treatment, follow-up period, and progression-free survival (PFS).
The institutional histopathological diagnoses were mostly based on the WHO 2007 criteria, and the diagnoses were adjusted to the recently revised WHO 2016 criteria incorporating molecular information. The molecular analyses were performed at each institution.
PFS was calculated from the date of initial surgery until the date of documented tumor progression or death, and the median PFS was estimated using Kaplan-Meier survival analysis. To determine the factors that affect PFS in HD patients with high-grade gliomas, univariate analyses were performed using Fisher exact tests to compare categorical variables. Analyses were performed using JMP 13.2.0 (SAS Institute Inc). AEs were evaluated using CTCAE version 4. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
Table 1 shows the clinical characteristics of the 7 patients. All 7 patients were men, and the average age was 66 years. None of the 7 patients had evidence of previous hepatitis B infection. The histopathological diagnoses were isocitrate dehydrogenase (IDH) wild-type glioblastoma in 4 cases, not other specified (NOS) glioblastoma in 2 cases, and IDH-mutant anaplastic astrocytoma in 1 case. The O(6)-methylguanine-DNA- methyltransferase (MGMT) promoter methylation was found in 6 patients and was not tested in the remaining 1. Six of the 7 patients underwent a surgical resection, and 4 of the 7 patients received the standard Stupp protocol1 as adjuvant therapy. In Case 1, 5-day TMZ was administered concomitantly with radiotherapy, and the dose of TMZ during the adjuvant phase was escalated by 10 mg/m2 course by course. In Case 5, the irradiation rate was reduced to 48 Gy at the patient’s request because he felt irritating pain around the wound and was unable to stay still in the irradiation facility.
Table 1.
Clinical Characteristic of the 7 Patients on Hemodialysis Treated by Radiochemotherapy With Temozolomide
| Case | Sex | Age, y | Location | Resection Rate | MGMT Methylation | Pathology | Initial Concomitant Chemoradiotherapy | Timing of TMZ Administration in Relation to HD | Protocol in Maintenance Therapy | KPS Before Concomitant Therapy | PFS (mo.) | Survival Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | FL | GTR | Yes | Glioblastoma, NOS | 5 days TMZ (100 mg/m2) oral + radiation 56 Gya | After HD | 5 days TMZ (100 mg/ m2) every 28-day cycle, 4 cyclesa | 30 | 6 | Death at 9 mo. |
| 2 | M | 68 | P | GTR | Not tested | Glioblastoma, NOS | 42 days TMZ (75 mg/m2) oral + radiation 60 Gy | After HD | 5 days TMZ (150 mg/ m2) every 28-day cycle, 11 cycles | 90 | 12< | No recurrence |
| 3 | M | 66 | O | GTR | Yes | Glioblastoma | 42 days TMZ (75 mg/m2) oral + radiation 60 Gy | After HD | 5 days TMZ (150 mg/ m2) every 28-day cycle, 8 cycles | 90 | 9< | No recurrence |
| 4 | M | 67 | FL | STR | Yes | Anaplastic astrocytoma | 42 days TMZ (75 mg/m2) oral + radiation 60 Gy | After HD | 5 days TMZ (150 mg/ m2) every 28-day cycle, 5 cycles | 50 | 6< | No recurrence |
| 5 | M | 57 | C | STR | Yes | Glioblastoma | 36 days TMZ (75 mg/m2) oral + radiation 48 Gyb | After HD | 5 days TMZ (150 mg/ m2) every 28-day cycle, 3 cycles | 50 | 6.5 | Death due to bacterial pneumonia |
| 6 | M | 64 | P | GTR | Yes | Glioblastoma | 42 days TMZ (75 mg/m2) oral + radiation 60 Gy | Before HD | 5 days TMZ (150 mg/m2) every 28-day cycle, 12 cycles | 90 | 40.7 | Death due to asthenia |
| 7 | M | 80 | T | PR | Yes | Glioblastoma | 10 days TMZ (75 mg/m2) oral + radiation 60 Gyc | Before HD | – | 50 | 4 | Death due to various infections |
Abbreviations: C, cerebellum; F, female; FL, frontal lobe; GTR, gross total resection; HD, hemodialysis; M, male; MGMT, O(6)-methylguanine-DNA-methyltransferase; mo., months; N, not otherwise specified; P, parietal lobe; O, occipital lobe, OS, overall survival; PFS, progression-free survival; PR, partial resection; STR, subtotal resection; T, temporal lobe; TMZ, temozolomide; y, years.
aIn Case 1, 5-day TMZ was administered concomitantly with radiotherapy, and the dose of TMZ during adjuvant phase was escalated by 10 mg/m2 each time course by course.
bIrradiation was suspended at 48 Gy at the patient’s request because he felt irritating pain around the wound and was unable to stay still in the irradiation facility.
cTMZ administration was suspended after day 10 of the concomitant phase because of skin eruption.
None of the patients received bevacizumab, and a carmustine wafer was implanted in one patient during surgery. In Case 7, the patient discontinued taking TMZ after the 10th day of the concomitant phase because of the occurrence of skin rash. HD may affect the dose of blood concentration because the molecular weight of TMZ and metabolic products are smaller than the permeability transition pore. With respect to the timing of TMZ uptake in 1 day, 5 patients received TMZ after HD, and 2 patients received TMZ in the early morning before HD. One patient with NOS glioblastoma underwent radiotherapy (radiation dose, 56 Gy) with oral concomitant TMZ (100 mg/m2) for 5 days followed by adjuvant 5-day TMZ (110 mg/m2) every 28 days as the initial course of maintenance therapy with an increasing dosage of 10 mg/m2 for each of 4 cycles. Patients on HD or those with severe renal disease who underwent chemotherapy usually received a 50% dose reduction of the alkylating agents.5 Case 1 was started with a 50% dose to avoid potential hematological toxicity. The dose was increased by 10 mg/m2 in each cycle as long as patients did not show any adverse side effects. In Case 6, an MRI taken 2 years after radiochemotherapy revealed progression of white-matter T2/fluid-attenuated inversion recovery hyperintensities. Clinically this translated to impairment in the patient’s daily physical activities. Although the last MRI, which was obtained 3 years after surgery, showed no tumor recurrence, he was hospitalized because of a fall, subsequently became bedridden, and, 5 months later, succumbed to aspiration pneumonia (Table 1).
One patient presented with tumor progression during the maintenance chemotherapy during the concomitant radiochemotherapy (Case 5), and one patient presented with tumor progression during the concomitant radiochemotherapy(Case 1) (Table 1). The median PFS was 12 months (range, 4-40.7 months).
Severe AEs in the 7 HD patients during the concomitant radiochemotherapy with TMZ are shown in Table 2. During the concomitant radiochemotherapy, 2 patients had Grade 3 and 4 lymphocytopenia, respectively, and 1 patient had Grade 3 thrombocytopenia. In this series, Cases 2, 3, and 5, who presented with lymphocytopenia Grade 3, spontaneously recovered (ie, improvement of lymphocyte counts, as stated below) during the concomitant therapy. In Case 4, the patient showed lymphocytopenia Grade 4, and TMZ therapy was temporarily discontinued to allow recovery from myelotoxicity, then restarted without any alteration in the chemotherapeutic dose.
Table 2.
Severe Adverse Events (Grades 3-5) During Treatment With Temozolomide in the 7 Patients on Hemodialysis
| Concomitant Phase | Maintenance Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Case | Neutropenia | Lymphopenia | Thrombocytopenia | Nonhematological | Neutropenia | Lymphopenia | Thrombocytopenia | Nonhematological |
| 1 | None | None | None | None | None | None | None | None |
| 2 | None | Yes (Grade 3) | Yes (Grade 3) | None | Yes (Grade 3) | Yes (Grade 3) | None | None |
| 3 | None | Yes (Grade 4) | None | None | None | Yes (Grade 3) | None | None |
| 4 | None | None | None | None | None | None | None | None |
| 5 | None | Yes (Grade 3) | None | None | None | None | None | Pneumonia (Grade 5) |
| 6 | None | Yes (Grade 4) | None | None | None | None | None | Leukoencephalopathy (Grade 3) |
| 7 | None | None | None | Skin rash (Grade 3) | NAa | NA | NA | NA |
Abbreviation: NA, not available. Common Terminology Criteria for Adverse Events version 4.
aAdjuvant 5-day temozolomide was not administered in Case 7.
With regard to nonhematological toxicity, 1 patient had Grade 2 pneumonia, and 1 patient had Grade 3 skin rash, which led to the discontinuation of daily TMZ. During the maintenance therapy, Grade 3 lymphocytopenia in 2 patients and Grade 3 neutropenia in 1 patient were observed; severe thrombocytopenia was not observed in any of the patients. After 3 courses of adjuvant TMZ therapy, 1 patient developed bacterial pneumonia caused by methicillin-resistant Staphylococcus aureus and eventually died of it. Another patient developed Grade 3 leukoencephalopathy. There was no difference in the frequency and degree of AEs with regard to the timing of TMZ administration (before HD vs after HD) according to the statistical analysis. Median KPS and modified Rankin scale before the treatment were 50 and 2.5, and 70 and 3 after the concomitant radiochemotherapy, respectively.
Case Presentation (Case 3)
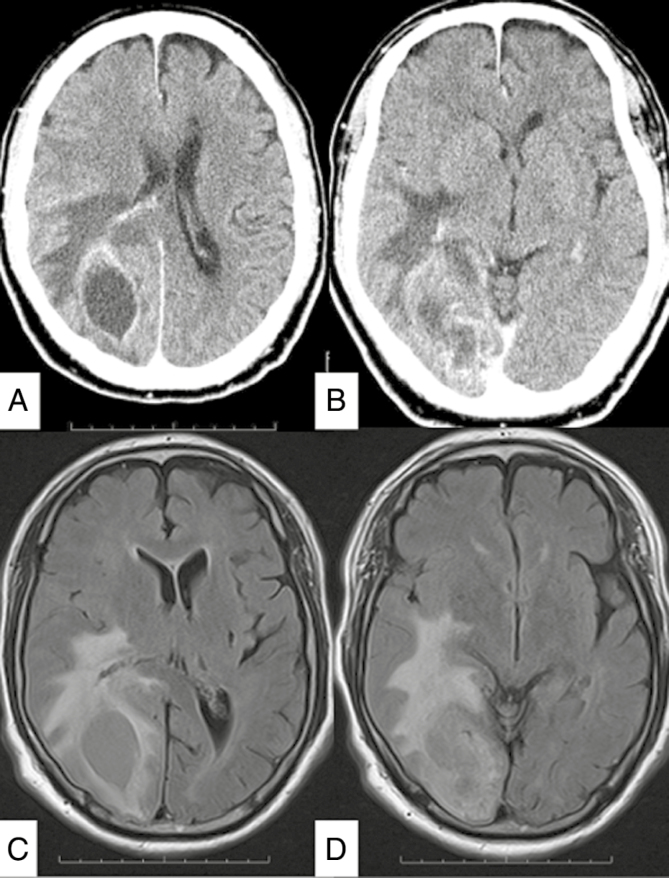
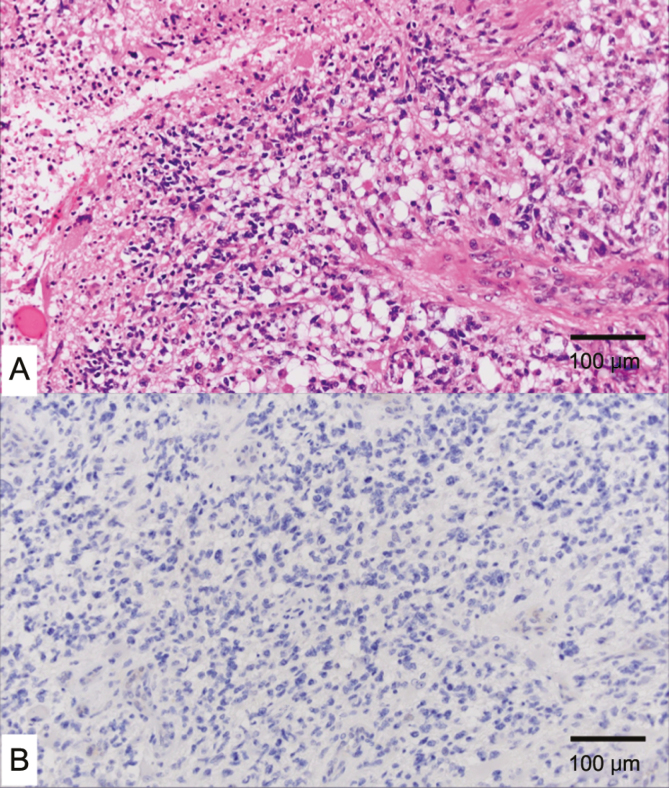
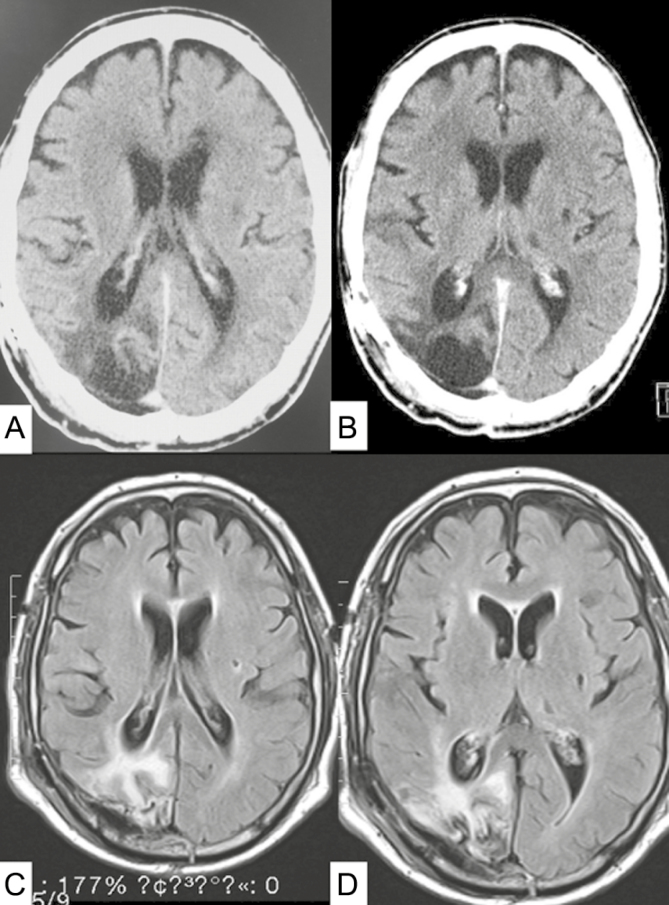
A 66-year-old man presented to the emergency room following a general convulsion during HD. CT scan and MRI showed an enhanced cystic lesion with edema in the right occipital lobe (Fig. 1). The patient presented with a left lateral homonymous hemianopia and had a KPS of 80. He underwent a gross total resection of the tumor. The histopathological diagnosis was IDH wild-type glioblastoma (Fig. 2). The promoter region of the MGMT gene was methylated by a methylation-specific polymerase chain reaction.6 The patient received concomitant daily TMZ (75 mg/m2), the same dose as non-HD patients, for 42 days with radiotherapy (radiation dose, 60 Gy per 30 fractions). On the days of HD, TMZ was administered after HD. During the concomitant radiochemotherapy, the patient presented with Grade 3 and 4 lymphocytopenia from day 28 to day 42. The TMZ therapy was temporarily discontinued for 3 days, to be resumed at the same dose only after lymphocytic recovery to at least Grade 3 level was documented. The patient completed a total of 42 days of TMZ administration, excluding the days of temporal discontinuation, hence it was not prolonged. After the completion of radiochemotherapy, the patient was treated with adjuvant TMZ therapy by the usual 5- per 28-day schedule (150 mg/m2, days 1-5) without decreasing the dose for a total of 12 courses. During the adjuvant TMZ monotherapy, the patient presented with Grade 3 lymphocytopenia; however, decreasing the TMZ dose was not required. At the end of the adjuvant TMZ therapy, the patient’s KPS was 90 (visual field deficit), and there was no evidence of tumor recurrence 9 months after the initial surgery (Fig. 3).
Fig. 1.
A and B, Preoperative axial contrast-enhanced CT scans demonstrate a heterogeneously enhanced tumor with cyst in the right occipital lobe. C and D, Axial, MRI fluid-attenuated inversion recovery–weighted image demonstrates the tumor in the right occipital lobe with cyst and brain edema around the tumor.
Fig. 2.
A, Histologic evaluation by hematoxylin-eosin staining showing a polymorphic glia cell increased in higher density and higher vascularity with necrosis. B, Tumor cells were positive for isocitrate dehydrogenase 1/2. A and B, Scale bar = 100 μm.
Fig. 3.
A and B, Postoperative axial contrast-enhanced CT scans demonstrate total tumor resection and no enhanced lesion. C and D, Axial MRI fluid-attenuated inversion recovery–weighted image demonstrates gross total tumor resection without brain injury.
Discussion
TMZ is primarily metabolized by hydrolysis, and TMZ and its metabolic products are mainly excreted in the urine. The time from administration to maximum blood dose concentration is 1 hour, and the time to half dose is 2 hours.7 The radioactivity of orally administered TMZ is recovered in the urine as TMZ (5.6%) and AIC (12%); therefore, possible AIC accumulation may enhance TMZ-associated AEs in patients with chronic kidney disease.4 However, TMZ has not been studied in HD patients, and TMZ prescription information with regard to information on TMZ toxicity and pharmacodynamics is lacking in those patients.
In Table 3, studies in patients with adequate renal function who underwent initial chemoradiotherapy with TMZ were reviewed. In the literature, the rates of hematotoxicity of patients with adequate renal function who received concomitant initial TMZ therapy were 19.3% to 43% and 1% to 9.8% for greater than or equal to Grade 2 and greater than or equal to Grade 3 neutropenia, 78.9% to 94.4% and 22% to 61% for greater than or equal to Grade 2 and greater than or equal to Grade 3 lymphocytopenia, and 3% to 28% and 1% to 7.2% for greater than or equal to Grade 2 and greater than or equal to Grade 3 thrombocytopenia9–14 (Table 3). On the other hand, the rates of greater than or equal to Grade 2 and greater than or equal to Grade 3 neutropenia were 14% and 0%, greater than or equal to Grade 2 and greater than or equal to Grade 3 lymphocytopenia were 85% and 57%, and greater than or equal to Grade 2 and greater than or equal to Grade 3 thrombocytopenia were 14% and 14%, respectively, in this series of HD patients receiving concomitant initial TMZ therapy. Therefore, although the series comprises a smaller number of patients, the risk of hematotoxicity in HD patients was similar, that is, within the range reported in previous studies, to patients with adequate renal function. Indeed, there was no severe hematotoxicity that led to TMZ discontinuation, regardless if concomitant daily TMZ was completed with radiotherapy (radiation dose, 48-60 Gy) in 5 of the 7 cases.
Table 3.
Hematotoxicity in the Present Series in Comparison With Those in Nonhemodialysis Cases During Initial Chemoradiotherapy, as Cited in the Literature
| Performance Status | No. of Cases | Neutropenia ≥ Grade 3 (%) | Lymphocytopenia ≥ Grade 3 (%) | Thrombocytopenia ≥ Grade 3 (%) | |
|---|---|---|---|---|---|
| Present study | 7 | 0 | 57 | 14 | |
| Stupp et al1 | PS = 0, 1, 2 | 284 | 4 | NA | 3 |
| Kobayashi et al8 | KPS > 50 (84%) KPS < 50 (16%) | 43 | 7 | 61 | NA |
| Kocher et al12 | KPS > 50 | 81 | 1 | 46 | 1 |
| Nishikawa et al13 | KPS > 70 | 32 | 3 | 22 | 6 |
| Clarke et al14 | KPS > 60 | 85 | 13 | 23.5 | 7 |
| Wick et al15 | KPS > 60 | 195 | 8.2 | 23.6 | 7.2 |
| Gilbert et al16 | KPS > 60 | 369 | 9.8 | 28.9 | 7 |
Abbreviations: NA, not available; PS, Performance Scale (WHO).
The risk factors for lymphocytopenia are female sex, loss of weight, sulfamethoxazole-trimethoprim combination,8 and the number of lymphocytes (<1200/μl) before TMZ treatment (prelymph).9
Although the incidence of neutropenia and thrombocytopenia in the present series was within the range reported in the literature with non-HD patients, 2 of the 7 cases (Cases 3 and 5) had bacterial pneumonia. Case 3 showed bacterial pneumonia in the concomitant initial therapy, not caused by P carinii, during the period of lymphocytopenia without neutropenia, that was treated with antibiotics for 1 week. This pneumonia cannot correlate with lymphocytopenia. Case 5 showed either severe neutropenia or lymphocytopenia before the infection caused by bacterial pneumonia, and pneumonia caused the deterioration of the patient’s performance status. Ichie et al reported that in their series of patients receiving concomitant initial TMZ therapy with radiation, patients older than 65 years demonstrated a 16.7% incidence rate of greater than or equal to Grade 3 thrombocytopenia.10 In our hemodialysis series, 4 of the 7 patients were older than 65 years, and 1 of the 4 patients experienced Grade 3 thrombocytopenia. Therefore, generally, the frequency and degree of myelosuppression do not increase in HD patients with high-grade gliomas.
The molecular weights of TMZ, AIC, and MTIC are 194.15 Da, 338.21 Da, and 168.16 Da, respectively. Generally, in HD patients, the factors that influence the elimination rate of the drugs include the following: the type of dialysis membrane, molecular weight of the drugs, volume of distribution of the drugs, protein-binding rates of the drugs, other dialysis conditions, and the molecular weight of the substance that can pass through the HD membrane, which is less than 500 Da.11 Such factors should be taken into consideration.
As medical technologies progress and the life span of people increases, HD patients have the chance to live longer and neurosurgeons will have a greater chance of seeing HD patients with high-grade gliomas. The data reported by the Japanese Society for Dialysis Therapy in 2016 demonstrated that 21.9% of deaths resulted from infection in HD patients. In the present series, 2 of the 7 patients (28.5%) died of infection despite having no direct correlation to myelosuppression, and physicians need to be more careful in preventing and treating infectious diseases in HD patients with high-grade gliomas.
Although this is a small-number case series, these results raise important concerns regarding serious clinical conditions and will lead to multicentered, randomized control studies in the future.
Conclusions
The administration of the regular treatment dose of TMZ to HD patients, supplemented by careful observation of hematological toxicity and prophylactic use of sulfamethoxazole-trimethoprim combination, is feasible.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. None declared.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Highlight of Prescribing Information: Temozolomide. USA; 2017:1–17. [Google Scholar]
- 3. Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992;65(2):287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oikawa M, Ito T, Takahashi S, et al. Experience with temozolomide for two patients with malignant glioma. J Hokkaido Brain Res Found. 2011;22(1):17–20. [Google Scholar]
- 5. Okamoto M, Nobori T, Matsuyama S, Ushigome M, Okajima H, Yoshimura R. Malignant tumor—how to use the medication for hemodialysis patients. Jin to Tōseki [Kidney and Dialysis]. 2011;70(4):671–675. [Google Scholar]
- 6. Ezaki T, Sasaki H, Hirose Y, Miwa T, Yoshida K, Kawase T.et al. Molecular characteristics of pediatric non-ependymal, non-pilocytic gliomas associated with resistance to temozolomide. Mol Med Rep. 2011;4(6):1101–1105. [DOI] [PubMed] [Google Scholar]
- 7. Aoki T, Nishikawa R, Mizutani T, et al. Pharmacokinetic study of temozolomide on a daily-for-5-days schedule in Japanese patients with relapsed malignant gliomas: first study in Asians. Int J Clin Oncol. 2007;12(5):341–349. [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi S, Shida T, Toyoguchi T, Sakurada K, Shiraishi T. Risk analysis of lymphocytopenia with temozolomide radiochemotherapy. Iryo Yakugaku [Japanese Journal of Pharmaceutical Health Care and Sciences]. 2012;38(8):471–478. [Google Scholar]
- 9. Ishikawa E, Yamamoto T, Sakamoto N, et al. Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo). 2010;50(8):638–644. [DOI] [PubMed] [Google Scholar]
- 10. Ichie T, Takahashi H, Yamaseki C, et al. Analysis of factors influencing thrombocytopenia associated with concomitant temozolomide and radiotherapy. Nichibyoshi. 2015;51(5):545–548. [Google Scholar]
- 11. Chennavasin P, Brater DC. Nomograms for drug use in renal disease. Clin Pharmacokinet. 1981;6(3):193–214. [DOI] [PubMed] [Google Scholar]
- 12. Kocher M, Kunze S, Eich HT, Semrau R, Müller RP.et al. Efficacy and toxicity of postoperative temozolomide radiochemotherapy in malignant glioma. Strahlenther Onkol. 2005;181(3):157–163. [DOI] [PubMed] [Google Scholar]
- 13. Nishikawa R, Shibui S, Maruno M, et al. Efficacy and safety of monotherapy with temozolomide in patients with anaplastic astrocytoma at first relapse—a phase II clinical study [article in Japanese]. Gan To Kagaku Ryoho [Jpn J Cancer Chemother]. 2006;33(9):1279–1285. [PubMed] [Google Scholar]
- 14. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27(23):3861–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 16. Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: a randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM). J Clin Oncol. 2011;29(suppl 15):2006. [Google Scholar]