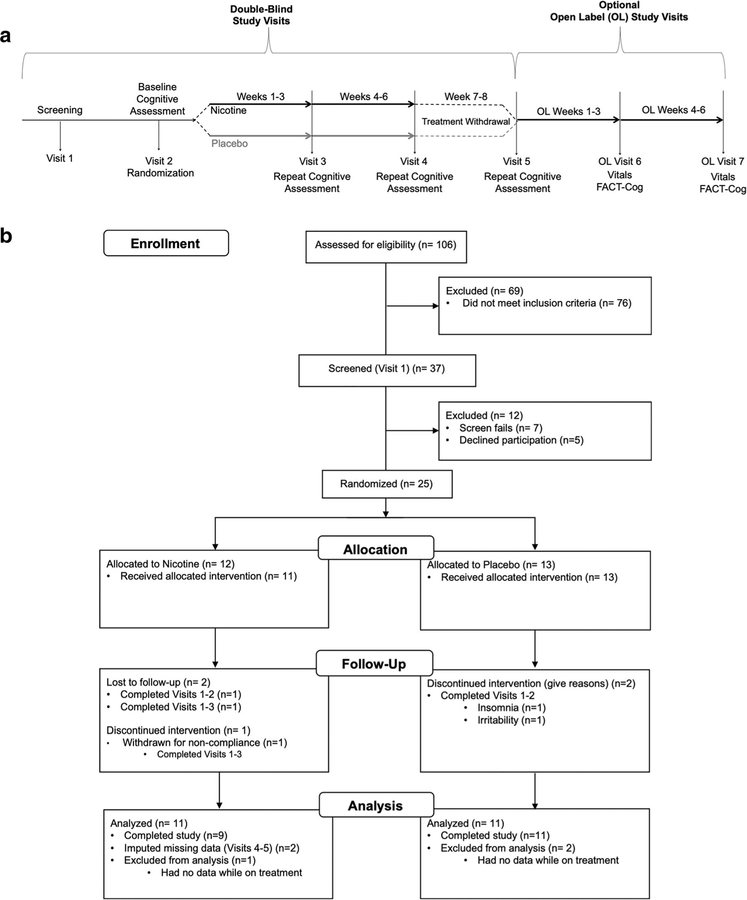
Fig. 1.
Study design and participants enrollment. a Overall pCRCI study design. The study consisted of two phases, a double-blind portion and an optional open-label portion. In the double-blind portion of the study, participants were first screened (visit 1) to determine study eligibility. Once cleared for the study, participants completed a baseline visit (visit2) and were then randomized (50/50) to receive either transdermal nicotine or placebo patches. Participants then repeated their baseline cognitive assessment at visits 3 and 4 following 3 weeks and 6 weeks on patches, respectively. After completing 6 weeks on patches, participants went off patches for 2 weeks, then repeated their baseline cognitive testing at their final double-blind study visit (visit 5). At the end of the double-blind, placebo-controlled 8-week study, participants had the option to take part in the open-label portion of the study for an additional 6 weeks. For all open-label visits (visits 6 and 7), only vitals were collected and one subjective test was completed. b Participant enrollment. Of the 106 people pre-screened for the study, 37 were screened at visit 1. Of the 37 screened at visit 1, 25 people passed screening criteria and were randomized to treatment after completing visit 2. Twelve were randomized to nicotine treatment (9 completers, 11 with usable data) and 13 were randomized to placebo treatment (11 completers)