Abstract
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have clearly demonstrated their beneficial effect in diabetic kidney disease (DKD) on top of the standard of care [blood glucose control, renin–angiotensin system blockade, smoking cessation and blood pressure (BP) control], even in patients with overt DKD. However, the indication of this drug class is still blood glucose lowering in type 2 diabetic patients with estimated glomerular filtration rate >45 mL/min/1.73 m2. Based on the new evidence, several scientific societies have emphasized the preferential prescription of SGLT2i for patients at risk of heart failure or kidney disease, but still within the limits set by health authorities. A rapid positioning of both the European Medicines Agency and the US Food and Drug Administration will allow patients with overt DKD to benefit from SGLT2i. Clinical experience suggests that SGLT2i safety management may in part mirror renin–angiotensin blockade safety management in patients with overt DKD. This review focuses on the rationale for an indication of SGTL2i in DKD. We further propose clinical steps for maximizing the safety of SGLT2i in DKD patients on other antidiabetic, BP or diuretic medication.
Keywords: chronic renal failure, diabetic kidney disease, ESRD, SGLT2 inhibitors, type 2 diabetes
INTRODUCTION
Diabetic kidney disease (DKD) is the most frequent cause of end-stage kidney disease (ESKD) in developed countries [1–3]. Until 2016, the mainstay of DKD treatment was renin–angiotensin system (RAS) blockade [4]. From 2016, sodium-glucose cotransporter 2 inhibitors (SGLT2i) have clearly demonstrated beneficial effects in DKD on top of the standard of care, namely blood glucose control, RAS blockade, smoking cessation and blood pressure (BP) control [5–9]. Initially, SGLT2i was developed to lower plasma glucose in type 2 diabetes mellitus (T2DM) patients, but large randomized controlled trials (RCTs) have demonstrated both renal and cardiovascular protection in T2DM beyond the benefits derived from improved glycaemic control.
The renoprotective effects of SGLT2i were first observed in three large trials evaluating cardiovascular safety: Empagliflozinn, cardiovascular, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME), Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes (CANVAS Programme), and Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes (DECLARE-TIMI 58). In these studies, SGLT2i reduced albuminuria progression, prevented glomerular filtration rate (GFR) decline and decreased the incidence of renal replacement therapy in T2DM patients without overt DKD [5, 6, 8]. Three further trials address the kidney effects of SGLT2i on patients with overt DKD: Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy (CREDENCE), Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (DAPA-CKD), and the Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY). In April 2019, the CREDENCE trial's striking results on kidney and heart protection were published [7]. In concordance with DAPA-CKD results, some authors suggest the potential use of SGLT2i on top of RAS blockade in the early stages of DKD, when T2DM patients mainly exhibit hyperfiltration and mild albuminuria. With this approach, one expects that the cardiorenal protective effect of this treatment will be offered as prevention and not delayed to the already established DKD [10]. However, further studies in pre-diabetic patients are needed to confirm this hypothesis.
There were initial safety concerns as SGLT2i adverse drug reactions (ADRs) included acute kidney injury (AKI), diabetic ketoacidosis (DKA), amputations, urinary and genital tract infections (UTIs), bladder cancer and bone fractures [11]. However, recent trials have downplayed these risks and the benefit/risk balance remains positive. Interestingly, in a pooled analysis of 11 Phase 3 RCTs, dapagliflozin use in patients with estimated GFR (eGFR) between 12 and 45 mL/min/1.73 m2 (CKD G3b–4) decreased the urinary albumin creatinine ratio (UACR), BP and body weight without modulating glycated hemoglobin (HbA1c) or increasing major side effects [10]. This observation supports the concept of positive renal and cardiac effects of SGLT2i independent from glycaemic control.
In this review, we describe the molecular mechanism involved in cardiorenal protection in DKD and the current indications for SGLT2i treatment in DKD patients according to the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), as well as suggestions by the scientific community to update the indication to include overt DKD. We also propose clinical practice recommendations for the safe use of SGLT2i in DKD patients on other antidiabetic, hypertension and diuretic treatments.
NEW MOLECULAR MECHANISMS IN CARDIORENAL PROTECTION WITH SGLT2 INHIBITION
SGLT2i have become a promising option to treat T2DM as they decrease glycaemia and improve insulin resistance without producing severe hypoglycaemia [12]. In a meta-analysis published by Yang et al. [13], SGLT2i was demonstrated to reduce HbA1c and insulin dosage both in types 1 and 2 diabetic patients. This decrease in insulin doses could reflect an improvement in insulin resistance, which is a key mediator of type 2 diabetes [14]. Furthermore, it might in part explain the weight loss induced by this drug class. SGLT2i additionally reduces cardiovascular complications and slows DKD progression [5, 6, 8, 15–17]. Diverse experimental and clinical studies suggest that the cardiorenal protection exerted by SGLT2i may be explained by mechanisms independent of glycaemia and BP control.
SGLT2 is a glucose/sodium (Na+) cotransporter located at the apical membrane of proximal tubular cells that drives glucose into cells together with Na+. It is responsible for the reabsorption of 90% of the filtered glucose load [18–20]. In diabetic patients, the increase in filtered glucose enhances SGLT2 activity, which worsens glycaemic control and promotes Na+ loading, impairing BP control [21]. Moreover, proximal tubular Na+ reabsorption results in decreased Na+ availability at distal tubules and reduces macula densa adenosine signalling to afferent arterioles. The signalling reduction leads to afferent arteriole vasodilation, increases intraglomerular pressure and causes hyperfiltration. Na+/H+ exchanger isoform 3 (NHE3) is a proximal tubule Na+ transporter closely regulated by glucose metabolism and SGLT transporters, which makes it sensitive to SGLT2i [22]. In Otsuka Long-Evans Tokushima Fatty diabetic rats, empagliflozin decreased the tubular expression of NHE3, the Na+–K+–2Cl− cotransporter and the epithelial Na+ channel as compared with untreated littermates [23]. Therefore, SGLT2i lowers glycaemia and additionally promotes natriuresis by inhibiting both SGLT2 activity and other Na+ transporters such as NHE3. Thus, the natriuretic effect may contribute to cardiorenal benefits by lowering arterial pressure and re-activating renal tubulo-glomerular feedback signalling from the macula densa that decreases intraglomerular pressure and hyperfiltration. However, prior to SGLT2i use, no other diuretic demonstrated clear cardiorenal protective effects in diabetic patients although they reduced BP or volume overload. For this reason, we believe that the beneficial effects exerted by SGLT2i are the conjunction of diverse molecular mechanisms acting together. More studies on the bench side are needed to elucidate the involved pathways.
Beyond the antidiabetic and natriuretic properties, studies in non-diabetic mice and rats suggest that SGLT2i may exert additional direct kidney- or heart-protective actions [24–28]. Although in some settings SGLT2i did not prevent kidney damage, in most non-diabetic CKD models, they did decrease kidney fibrosis and inflammation [24–28]. The discrepant results may depend on the nature of the different insults tested: SGLT2i did not protect from chronic oxalosis [24] or subtotal nephrectomy [25], while it protected from unilateral ureteral obstruction [26], ischaemia–reperfusion [27] and protein overload [28]. In cultured human tubular cells (HK-2), transforming growth factor beta 1 (TGF-β1) upregulated SGLT2 and type IV collagen expression, and this was prevented by empagliflozin through nuclear factor-κB/Toll-like receptor 4 (NF-κB/TLR4) pathway inhibition, thus linking a key mechanism of DKD and non-DKD CKD progression to SGLT2 [29]. Furthermore, both mice and human glomerular cells also express SGLT2. Indeed, albumin upregulated SGLT2 in mice with protein-overload proteinuria, in albuminuric CKD patients and in cultured human podocytes [28]. Overall, the available evidence of benefit in non-diabetic models supports the existence of glucose-independent nephroprotective effects, and this hypothesis will be addressed in RCTs [26–30].
In search of further molecular targets and downstream mediators of SGLT2i effects, the canagliflozin mechanism of action (MoA) network was explored combining cultured tubular cell transcriptomics profiles and protein expression data from the scientific literature. This identified 44 proteins related to the canagliflozin MoA molecular model that overlapped with proteins in the DKD network model, and 10 of them were involved in DKD progression [31]. Assessment of these in plasma from diabetic patients with UACR > 1.7 mg/mmol included in the efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU trial) identified four candidate factors [tumour necrosis factor receptor 1 (TNFR1), interleukin (IL)-6, matrix metalloproteinase 7 (MMP-7) and fibronectin 1 (FN1)] that were decreased by canagliflozin, supporting the concept that SGLT2i may modulate inflammatory and fibrosis processes in DKD [31, 32]. In addition, the effects of two SGLT2i (empagliflozin and canagliflozin) and TGF-β1 on gene expression in two human proximal tubular cell lines were studied [33]. All 94 genes, mainly related to extracellular matrix organization, which were upregulated by TGF-β1 and downregulated by the two SGLT2i were identified. Three of these genes [thrombospondin 1 (THBS1), tenascin C (TNC) and platelet-derived growth factor subunit B (PDGF-B)] related to renal fibrosis and CKD progression were then validated on mRNA expression in both cell lines, proving that SGLT2i is able to suppress these genes.
Cardioprotection by SGLT2i has been widely attributed to the improved glycaemia and BP control [34]. However, a direct effect on the heart cannot be disregarded since there is an ongoing controversy on whether SGLT2 is expressed in the heart [35–39]. Recent data support direct effects on the heart since empagliflozin improved high glucose-induced cardiomyocyte dysfunction [40]. Empagliflozin also ameliorated cardiac function in pressure overload-induced heart failure in mice [41] and delayed ischaemic contracture in isolated mouse hearts in an insulin-dependent manner [42]. This last effect may depend on direct inhibition of the Na+/H+ transporter NHE1 as empagliflozin interacts with its Na+-binding domain [42, 43]. NHE1 and NHE3 belong to the same family of Na+/H+ transporters. NHE1 is virtually ubiquitously expressed and is responsible for intracellular pH maintenance, while NHE3 expression is restricted to epithelial tissues where it also contributes to transepithelial transport [44]. As mentioned before, in kidney tubules, NHE3 activity depends on SGLT2-mediated glucose transport [22]. It may be speculated that a similar mechanism applies to NHE1 in the heart [22, 40]. Thus, cardioprotection by SGLT2i may also depend on direct inhibition of local SGLT2. Finally, empagliflozin increased glucose transporter-1 (GLUT-1) mediated glucose transport in isolated cardiomyocytes [45], and this could also contribute to SGLT2i cardioprotection.
In summary, there is evidence that SGLT2i exert pleiotropic tissue-protective effects via different mechanisms (Supplementary data, Figure S1): (i) reduction of glycaemia, BP and volume overload; (ii) SGLT2 inhibition in kidney tubules leading to improved metabolic control, natriuresis and decreased hyperfiltration; (iii) local SGLT2 inhibition in other organs, such as the heart [40, 41] or the pancreas [46]; and (iv) SGLT2-independent interference with Na+/H+ transporters.
WHEN TO START SGLT2 IN DIABETIC PATIENTS?
As of June 2019, regulatory and scientific society statements and recommendations on the indications and timing of SGLT2i prescriptions differ. This likely reflects the different processing timelines for the wealth of recent information from high cardiovascular risk and DKD RCTs (Table 1) [5–8, 15]. The EMA current indication for SGLT2i is the treatment of insufficiently controlled T2DM as an adjunct to diet and exercise, either as monotherapy when metformin is considered inappropriate due to intolerance or in addition to other medicinal products for the treatment of diabetes, although dapagliflozin may also be used in T1DM (Table 2) [47–51]. Recently, the EMA recognized the positive effect of dapagliflozin on cardiovascular events. However, the US FDA was the first to acknowledge the specific indications in T2DM patients with cardiovascular disease (CVD) to reduce major adverse cardiovascular events (MACEs) for canagliflozin and to reduce cardiovascular death for empagliflozin, based on RCT results (Tables 1 and 2) [47–51]. While the US FDA specifically warns against the use of any SGLT2i in T1DM, the EMA admits the use of dapagliflozin for this indication. Overall, the FDA appears to have moved faster than the EMA in response to cardiovascular safety trial results and clearly distinguishes between specific indications for each drug as per RCT results (Figure 1A). The rapid response is likely related to the FDA request for these trials, and an indication for the treatment of DKD is undergoing priority review. In contrast, the EMA does not state large differences between the three drugs either in indication or in the use according to eGFR data. There are additional differences between major regulatory agencies regarding SGLT2i contraindications for DKD patients with decreased eGFR (Table 3 and Figure 1B).
Table 1.
Key cardiovascular safety and CKD trials of SGLT2i
| Drug | Trial | Trial type | CVD (% of patients) | eGFR (mL/min/1.73 m2) | UACR (mg/g)a | MACE HR (95% CI) | Stroke/AMI HR (95% CI) | CV death HR (95% CI) | Heart failure hospitalization HR (95% CI) | Renal outcome HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Canagliflozin | CANVAS [6] | CV safety | 65.6 | 76.5 | 12.3 (6.65–42.1) | 0.86* (0.75–0.97) | Not different | 0.87 (0.72–1.06) | 0.67 (0.52–0.87) | 0.60b (0.47–0.77) |
| CREDENCE [7] | DKD | 50.4 | 56.2 | 927 (463–1833) | 0.80 (0.55–1.00) | Not different | 0.78 (0.55–1.00) | 0.61 (0.47–0.80) | 0.68c (0.54–0.86) | |
| Dapagliflozin | DECLARE-TIMI [8] | CV safety | 40.6 | 85.2 | Not available | 0.93 (0.84–1.03) | Not different | 0.98 (0.82–1.17) | 0.73 (0.61–0.88) | 0.53d (0.43–0.66) |
| Empagliflozin | EMPA-REG OUTCOME [5, 15] | CV safety | 99.1 | 74 | Not availablee | 0.86* (0.74–0.99) | Not different | 0.62 (0.49–0.77) | 0.65 (0.50–0.85) | 0.54f (0.40–0.75) |
AMI, acute myocardial infarction; MACE, composite of cardiovascular death, myocardial infarction or ischaemic stroke.
Median (interquartile range).
Composite of 40% reduction in eGFR, renal replacement therapy or renal death.
Dialysis for at least 30 days, kidney transplantation or eGFR < 15 mL/min/1.73 m2 for at least 30 days.
Composite of ≥40% reduction in eGFR to <60 mL/min/1.73 m2, end-stage kidney disease or death from renal cause.
UACR ≥ 30 mg/g in 40% of participants.
Doubling of serum creatinine with eGFR ≤ 45 mL/min/1.73 m2, initiation of renal replacement therapy or death from renal disease.
P < 0.05 for superiority.
Table 2.
Indications (EMA, FDA) or recommendations (ADA-EASD, ESC, EURECA-m and DIABESITY) for the use of SGLT2i
| Drug | EMA (as of June 2019) [47] | FDA (as of June 2019) [48] | ADA-EASD 2018 [50] | ESC 2016 [49] | EURECA-m and DIABESITY 2019 [51] | CREDENCE-based likely future suggestions | |
|---|---|---|---|---|---|---|---|
| SGLT2i | Canagliflozin | T2DM controla |
|
|
|
|
|
| Dapagliflozin | T2DM controlb | ? | |||||
| Empagliflozin | T2DMa control | ? | |||||
CREDENCE based likely future suggestions reflect the authors views regarding potential future guidelines of EMA indications based on the results of the CREDENCE trial. These should be considered hypothetical and may not reflect the eventual decisions by these bodies
Adults with insufficiently controlled T2DM as an adjunct to diet and exercise, either as monotherapy when metformin is considered inappropriate due to intolerance or in addition to other medicinal products for the treatment of diabetes.
As an adjunct to diet and exercise to improve glycaemic control in adults with T2DM.
To reduce the risk of MACE in adult patients with T2DM and established CVD.
Adults with insufficiently controlled T1DM as an adjunct to insulin in patients with body mass index ≥27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy.
To reduce the risk of cardiovascular death in adult patients with T2DM and established CVD.
American Diabetes Association-European Association for the Study of Diabetes (ADA-EASD), European Society of Cardiology (ESC), European Renal and Cardiovascular Medicine (EURECA-m) and Diabetes and Obesity in Renal Disease (DIABESITY) are European Renal Association (ERA-EDTA) working groups.
FIGURE 1.
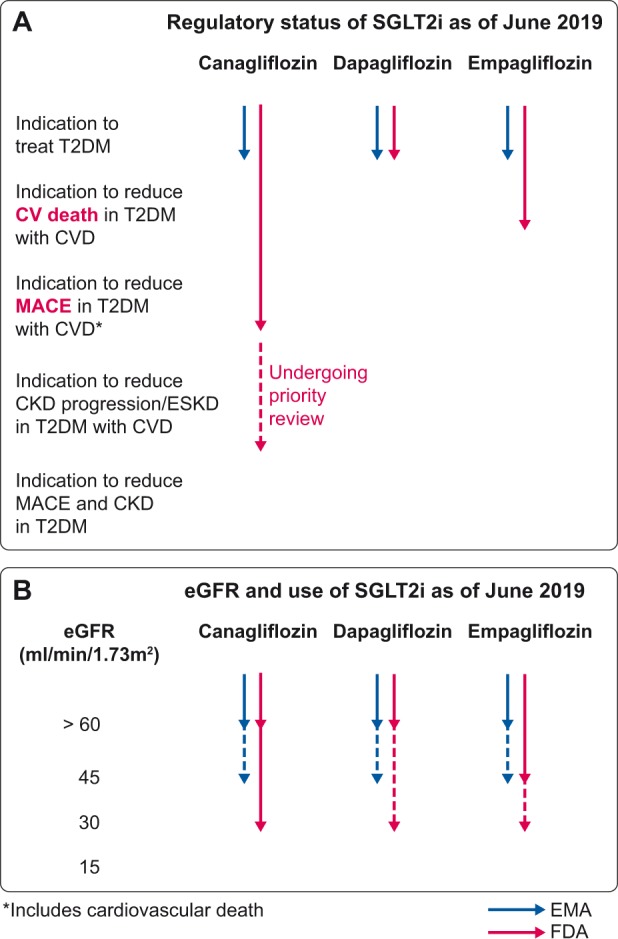
(A) Regulatory status regarding indications of SGLT2i as of June 2019. The FDA differentiates between the indications of the three SGLT2i according to T2DM patient characteristics, while the EMA does not. In contrast, EMA allows the use of dapagliflozin in T1DM, while the FDA warns against the use of any of these drugs in T1DM. (B) Regulatory status regarding use in individuals with decreased eGFR of SGLT2i as of June 2019. Language such as ‘contraindicated’ or ‘should not be used’ has been considered equivalent. Discontinuous line represents the situation in which the drug was initiated prior to the deterioration of renal function. Based on the results of the CREDENCE trial [7], we anticipate that at least for canagliflozin, usage will be allowed in the near future up to the initiation of renal replacement therapy.
Table 3.
Use of SGLT2i in patients DKD: impact of eGFR
| Drug | EMA (as of May 2019) [47] | FDA (as of May 2019) [44] | ADA-EASD 2018 [50] | ESC 2016 [49] | EURECA-m and DIABESITY 2019 [51] | CREDENCE-based likely future suggestions | |
|---|---|---|---|---|---|---|---|
| SGLT2i | Canagliflozin |
|
Contraindicated if eGFR <30 |
|
No mention |
|
|
| Dapagliflozin |
|
? | |||||
| Empagliflozin |
|
? | |||||
CREDENCE based likely future suggestions reflect the author’s views regarding potential future guidelines of EMA indications based on the results of the CREDENCE trial. These should be considered hypothetical and may not reflect the eventual decisions by these bodies. eGFR in mL/min/1.73 m2. EMA, ADA-EASD, ESCEURECA-m and DIABESITY are ERA-EDTA working groups.
Since 2016, scientific societies have also suggested the role of SGLT2i in patients with T2DM and specific comorbidities. Table 2 summarizes some of the key position manuscripts. Overall, scientific societies recommend considering SGLT2is as preferential agents to treat T2DM on top of metformin when patients have atherosclerotic CVD, heart failure or DKD. Contrary to the FDA, which provides different regulatory advice for each drug according to specific cardiovascular safety trial results, scientific societies usually use the generic term ‘SGLT2i’ or ‘SGLT2i with evidence of cardiovascular or renal protection’. Additionally, given the striking benefit over heart failure hospitalization, the preferential use of SGLT2i for patients with heart failure is suggested or recommended, although this was not a primary outcome of cardiovascular safety trials. Scientific societies fall short of recommending the use of SGLT2i outside the eGFR limits provided by regulatory authorities (Table 3). It is likely that the range of eGFR at which canagliflozin can be prescribed will expand in the near future, given the renal benefit observed in the CREDENCE trial of canagliflozin versus placebo in patients with overt DKD, in which patients were enrolled with eGFR ≥30 mL/min/1.73 m2, and canagliflozin was not stopped until renal replacement therapy was initiated (Supplementary data, Figure S2A). We believe that this supports an indication of canagliflozin at any eGFR value in non-dialysis patients, always with the caveat that initiation of SGLT2i may decrease the baseline eGFR as it decreases hyperfiltration. In this regard, in T2DM patients with DKD, canagliflozin decreased the relative risk of a composite primary outcome of ESKD (dialysis, transplantation or a sustained estimated GFR of < 15 mL/min/1.73 m2), doubling of the serum creatinine level or death from renal or cardiovascular causes by 30% versus placebo [hazard ratio (HR) = 0.70; 95% confidence interval (CI) 0.59–0.82; P = 0.00001] [7]. More specifically, a hard component of the composite, the residual risk of ESKD was reduced to 2/100 patient-years of follow-up (Supplementary data, Figure S2B). Taken together with beneficial effects over pre-specified renal outcomes such as incident or worsening nephropathy (progression to macroalbuminuria, doubling of serum creatinine, initiation of renal replacement therapy or death from renal disease) and incident albuminuria in patients with milder or no evidence of DKD in cardiovascular safety trials [5, 17], overall data point to a nephroprotective effect at different stages of DKD that warrants preferential use of SGLT2i to treat T2DM patients from very early in the course of DKD, and even before its occurrence to prevent or delay it. The kidney outcomes success of SGLT2i trials for T2DM has set the stage for additional trials enrolling non-T2DM patients [30].
SGLT2 AND DKD: PROS AND CONS
Three large cardiovascular safety trials—EMPA-REG OUTCOME, the CANVAS programme and DECLARE-TIMI 58—demonstrated that SGLT2i reduced albuminuria progression, prevented GFR decline and decreased the incidence of renal replacement therapy [5, 8, 11, 15, 17, 52]. However, these were secondary endpoints and few patients had moderate-to-severe DKD (Table 1), so information on patients with A3 albuminuria or GFR <60 mL/min/1.73 m2 was limited. Two ongoing (DAPA-CKD and EMPA-KIDNEY) and one just completed (CREDENCE) RCTs test with different SGLT2i for primary renal endpoints in patients with overt DKD. The CREDENCE trial evaluated the impact of SGLT2 inhibition (canagliflozin 100 mg daily) against placebo in patients with T2DM and DKD defined as UACR >300 mg/g with eGFR from 30 to 90 mL/min/1.73 m2 [1, 7]. CREDENCE was stopped prematurely because the pre-specified efficacy criteria for early cessation were reached, thus providing evidence of renal and heart protection in a population partly excluded from prior RCTs [53]. Regarding safety, rates of adverse events were similar between groups, with the exception of DKA (2.2 versus 0.2 events per 1000 patient-years in canagliflozin and placebo patients, respectively) and genital mycotic infection—especially, in male patients—which were more frequent in the canagliflozin group.
Despite the increasing evidence of SGLT2i efficacy in DKD, the use of this drug class is still limited to patients with eGFR >45 mL/min/1.73 m2. This restriction may be ascribed to two main reasons: (i) SGLT2i were designed as antidiabetic drugs and their antidiabetic effects depend on the magnitude of glycosuria, which is limited in patients with decreased GFR [54]; and (ii) given the mechanism of action, there was no rationale for their use as antidiabetic agents in patients with an eGFR <45 mL/min/1.73 m2. Accordingly, they were not even tested in large numbers of patients with low eGFR, until now. Although exploratory trials had evaluated the efficacy and safety of SGLT2i in established DKD [54], their clinical impact was low, as the sample size was small, the duration short and renal outcomes were not assessed. Interestingly, a meta-analysis of 4000 patients with eGFR from 45 to 60 mL/min/1.73 m2 from 11 RCTs demonstrated consistent positive effects of dapagliflozin on BP, body weight, haematocrit, albuminuria, eGFR, bicarbonate, pulse pressure and uric acid. CREDENCE confirmed kidney and cardiovascular benefits for patients starting canagliflozin in overt DKD patients (G3a and G3b, eGFR >30 mL/min/1.73 m2) that maintained therapy up to the start of dialysis [7]. This evidence should be sufficient for the approval of the use of this drug class or at least of canagliflozin in patients with eGFR <45 mL/min/1.73 m2.
The overall safety record of SGLT2i in patients with moderate-to-severe DKD has been positive [17, 54]. Adverse events were more frequent in patients receiving higher doses of this drug class. However, in CREDENCE, a low canagliflozin dose of 100 mg/day was nephroprotective and safe, suggesting that lower SGLT2i doses achieved renal and heart protection in patients with overt DKD, with eGFR <45 mL/min/1.73 m2 (mean eGFR 56 mL/min/1.73 m2) [7].
SAFETY OF SGLT2i: IS THERE A CLASS EFFECT?
Potential ADRs of SGLT2i include AKI [55], DKA [56], foot and toe amputations [17], UTI and genital infections [57], bladder cancer [58] and bone fractures [59]. The most consistent associations have been with DKA, UTI and genital infections. From 2015 to 2018, the FDA has issued six warnings about ADRs linked to SGLT2i. A review on the safety profile of SGLT2i that selected 27 studies for risk analysis concluded that causality assessment disclosed a correlation between SGLT2i and DKA and UTI [60]. A systematic review and meta-analysis of RCT that included SGLT2i up to May 2018 [61] selected 109 reports on SGLT2i, mainly dapagliflozin, canagliflozin, empagliflozin and ipragliflozin. When compared with placebo, SGLT2i decreased the risk of AKI (RR = 0.59; 95% CI 0.39–0.89; I2 = 0.0%), while no difference was found for DKA (RR = 0.66; 95% CI 0.30–1.45, I2 = 0.0%), UTI (RR = 1.02; 95% CI 0.95–1.09, I2 = 0.0%) or bone fracture (RR = 0.87; 95% CI 0.69–1.09, I2 = 1.3%). Three studies reported on amputation, with one finding a significantly increased risk. Subgroup analysis did show an increased risk of UTI with dapagliflozin only (RR = 1.21; 95% CI 1.02–1.43, I2 = 0.0%), but no other analysis supported an increased risk of AKI, DKA, UTI or fracture. The authors concluded that evidence from RCTs did not suggest an increased risk of harm with SGLT2i as a class over placebo or active comparators with respect to AKI, DKA, UTI or fracture. However, wide CIs for many comparisons suggest limited precision, and, therefore, clinically important ADRs could not be ruled out.
In the CANVAS programme, 10 142 T2DM patients were randomly allocated to canagliflozin 100 or 300 mg/day or placebo [18]. Canagliflozin was associated with a significant doubling in the risk for amputations, mainly toe and metatarsal amputations (6.3 versus 3.4 cases per 1000 patient-years, HR = 1.97, 95% CI 1.41–2.75) [62]. The risk was similar for ischaemic and infective aetiologies and for 100 and 300 mg doses. Overall amputation risk was strongly associated with baseline history of prior amputation (major or minor) (HR = 21.31, 95% CI 15.40–29.49) and other established risk factors. No interactions between randomized treatment and participant characteristics explained the effect of canagliflozin on amputation risk. For every clinical subgroup studied, numbers of amputation events projected were smaller than the numbers of MACE averted (HR = 1.97). Additionally, no differences in the incidence of amputations were observed in the CREDENCE RCT in patients with DKD randomized to 100 mg/day canagliflozin. Interestingly, when CREDENCE was started the amputations warning regarding the CANVAS study was already released, and investigators were aware of this ADR. However, no differences regarding baseline peripheral artery disease between the two studies were observed. In the DECLARE study, comparing dapagliflozin versus placebo added to standard care in 17 160 patients followed for a median of 4.2 years, there were no differences in the incidence of amputations, stroke or fractures, with a significant increase of UTI or genital infections and DKA. Moreover, there was a lower risk of hypoglycaemia, AKI and bladder cancer in the dapagliflozin-treated group (Supplementary data, Tables S1 and S2) [8]. To clarify whether the amputation risk could be a class effect of SGLT2i, a thorough collection of prospective data on amputations and other foot-related complications is required in all ongoing and future long-term randomized clinical trials (e.g. DAPA-CKD, Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) and DECLARE with dapagliflozin; EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved) and EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced); and Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease (VERTIS CV) with ertugliflozin).
Recently, the use of SGLT2i has been also associated with Fournier gangrene, a rare urologic emergency characterized by necrotizing infection of the external genitalia, perineum and perianal region. The FDA identified 55 unique cases of Fournier gangrene in patients receiving SGLT2i between 1 March 2013 and 31 January 2019. Age ranged from 33 to 87 years; 39 were men and 16 were women. Time to onset after SGLT2i initiation ranged from 5 days to 49 months. All patients had DKA (n = 8), sepsis or septic shock (n = 9) and AKI (n = 4). Eight patients had faecal diversion surgery, two developed necrotizing fascitis of a lower extremity that required amputation and one required a lower extremity bypass procedure because of gangrenous toes. Three patients died. For comparison, the FDA identified 19 Fournier gangrene cases associated with other antidiabetic agents between 1984 and 31 January 2019: metformin (n = 8), insulin glargine (n = 6), short-acting insulin (n = 2), sitagliptin plus metformin (n = 2), and dulaglutide (n = 1). These patients ranged in age from 42 to 79 years; 12 were men and 7 were women. Two patients died [63]. These findings merit further research.
In conclusion, genital infections are probably the most frequent and documented ADR of the SGLT2i. Other potential ADRs are less consistent and variable data can be found in different RCTs and meta-analysis. Given the similar molecular structure of the available SGLT2i, it is plausible that ADRs observed until now are a class effect. The discrepancies observed in the published RCTs may be ascribed to the differences in patient characteristics as well as in evolving clinical experience with these agents rather than to differences between specific SGLT2i.
CLINICAL RECOMMENDATIONS FOR THE USE OF SGLT2 INHIBITORS IN DKD
A multifactorial intervention is still the best therapeutic approach to T2DM with or without DKD. These include implementing lifestyle interventions, promoting weight loss (healthy diet and exercise) in obese patients, reducing low-density lipoprotein cholesterol levels using statins, treating BP preferentially with RAS blockade and improving glycaemic control [51, 64]. For the latter objective, metformin is still considered the first pharmacological option if tolerated, although safety and prescribed doses should be revised if eGFR is 30–60 mL/min/1.73 m2 and the fact sheet recommends stopping the drug when eGFR falls below 30 mL/min/1.73 m2 [65], although this is a debated issue. Recent consensus documents have addressed the place of SGLT2i and glucagon-like peptide-1 receptor agonists (GLP-1RAs), in light of cardiovascular and renal outcomes trials [51, 64, 66]. These documents consider SGLT2i and GLP-1RAs as second-line therapy in both DKD with unmet glycaemic control (HbA1c >7%) and for controlled DKD patients (HbA1c <7%). Specifically, consensus documents recommend the use of SGLT2i in T2DM patients with established CVD or DKD. These changes shift the focus of this drug prescription from glycaemic control to improved kidney and cardiovascular outcomes.
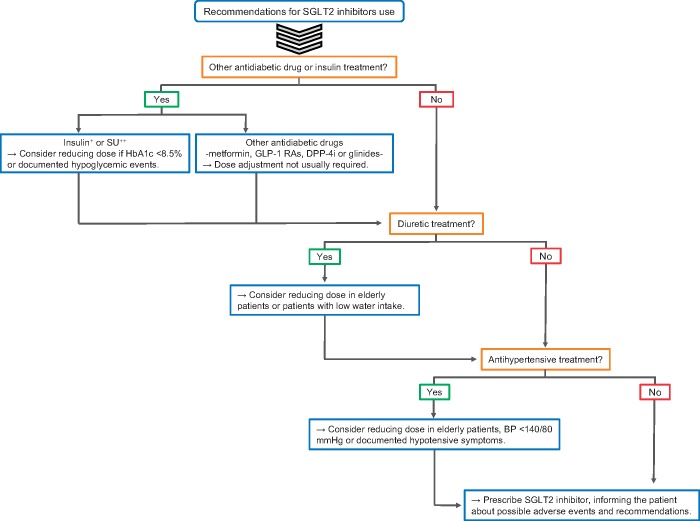
The mechanistic effect of this drug class, including natriuresis and glycosuria, guides the concomitant use of other drugs and suggests some preventive measures to increase safety when initiating SGLT2i treatment in patients with CKD on other antidiabetic drugs or diuretics. Thus, the lower antidiabetic efficacy of SGLT2i at low eGFR may require a combination with other antidiabetic agents [67]. Severe hypoglycaemia is rarely described with the use of SGLT2i and is usually associated with the add-on therapy to other hypoglycaemic drugs [54, 67]. To prevent hypoglycaemic events in DKD patients, other antidiabetic drugs should be titrated, reduced or withdrawn before starting SGLT2i (Figure 2). This recommendation is particularly important in DKD patients on sulphonylureas (SU) and/or on insulin, which is already associated with an increased risk of hypoglycaemia in CKD [54, 67]. Combination with other antidiabetic drugs, such as GLP-1RAs or metformin, has a much lower risk of hypoglycaemic events [69]. Because of the diuretic effect, doses of other diuretic medications should also be revised and in many cases decreased when starting SGLT2i in DKD patients (Figure 2). In elderly or frail patients, who may have a lower water intake or access, SGLT2i therapy should be carefully initiated and closely monitored.
FIGURE 2.
Recommendations before starting treatment with SGLT2i. +A decrease of 20% of the insulin dose is recommended, especially if there are previously reported hypoglycaemic events or the eGFR is <60 mL/min/1.73 m2. ++Sulphonylureas (SU) should be withdrawn if eGFR falls below 45 mL/min/1.73 m2. DPP-4i, dipeptidyl peptidase 4 inhibitor GLP-1 RAs, glucagon-like peptide-1 receptor agonists. Adapted from reference [68].
The routine risk management strategy for SGLT2i is very similar to that for RAS blockers, which is familiar for both patients and physicians. Thus, patients on SGLT2i treatment should be advised to temporarily discontinue the drug if acutely ill, especially in the presence of vomiting, diarrhoea or decreased water intake, to avoid dehydration and volume depletion. Similarly, SGLT2i should be transiently withdrawn during acute infections, fever, surgery and other insulin counter-regulatory states to prevent DKA [67, 70]. DKA is a rare but severe complication of SGLT2i treatment that, unlike classic DKA, is usually associated with mild-to-moderate serum glucose elevations. DKA may be precipitated by fasting, alcohol intake, excessive reduction of insulin doses, dehydration or acute processes, although some cases remain unexplained [71]. To avoid DKA, SGLT2i should be initiated at the lowest dose possible when blood ketone levels are <0.6 mmol/L [72]. Moreover, it is not advisable to start this drug class in patients with an already known low reservoir of endogenous insulin or low C-peptide. It is recommended that patients self-monitor capillary blood ketones when DKA is suspected, but urine ketones monitoring would be enough. SGLT2i therapy should be discontinued if ketone levels increase. Proper education is important for both the patient and the clinician to be fully aware of factors impacting ketone levels and the protocol to follow if ketone levels increase. In concordance, a nephrologist should suspect euglycaemic DKA when called as consultant in the emergency department for a severe metabolic acidosis with high anion gap in a diabetic patient under SGLT2i therapy. SGLT2i may be not initiated if hypovolaemia or peripheral vascular disease is present or in older frail patients.
CONCLUSIONS
In conclusion, emerging evidence derived from RCTs and meta-analyses point to cardioprotective and nephroprotective effects of SGLT2i that are maintained in patients with full-blown DKD with baseline eGFR up to 30 mL/min/1.73 m2. The use of at least one of these drugs, canagliflozin at the 100 mg/day dose, was safe and associated with benefits in DKD patients that maintain the drug up to the start of renal replacement therapy. Scientific societies have moved to recommend the preferential use of SGLT2i in patients with DKD. It is expected that regulatory authorities will increase the range of eGFR at which SGLT2i can be used, as well as modify the indications to include nephroprotection. In addition, the development of multidisciplinary teams, including a nephrologist, cardiologist, endocrinologist and primary care doctor for the management of high-risk type 2 diabetic patients (previous history of CVD or DKD), would improve the proper implementation of this class of anti-hyperglycaemic drugs in clinical practice. SGLT2i are generally safe, and some of the initial safety concerns have not been confirmed in more recent RCTs. In any case, both physicians and patients should be well aware of potential ADRs and instructed to discontinue the drug in certain circumstances. Given the protection afforded in patients with low eGFR, where the contribution of improved metabolic control is minimal, it is thought that the molecular mechanisms of cardiovascular and kidney protection expand beyond lowering serum glucose levels. In this regard, RCTs that explore cardiovascular and kidney protection even in patients without T2DM are ongoing.
Supplementary Material
FUNDING
FIS/Fondos FEDER (PI16/02057, PI17/00257, ISCIII-RETIC REDinREN RD016/0009), FRIAT, Sociedad Española de Nefrología, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM, and CIBER CV 16/11/00420.
CONFLICT OF INTEREST STATEMENT
A.O. has received speaker fees from Sanofi-Genzyme, Amgen, Fresenius Medical Care, Mundipharma, Amicus and Freeline. M.J.S. has received speaker fees or travel support from Otsuka, Menarini, Astrazeneca, Boehringer Ingelheim, Janssen, Mundipharma, Novartis, Eli Lilly, Esteve and Novonordisk. J.F.N.-G. has served as a consultant, and has received speaker fees or travel support from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Genzyme, Lilly, Novartis, Servier, Shire and Vifor Fresenius Medical Care Renal Pharma. J.L.G. has received fees for giving talks from: Astrazeneca, Boehringer Ingelheim, Janssen, Mundipharma, Novartis, Novonordisk, Otsuka and Vifor Pharma. A.M.C. has received speaker fees from Boëhringer-Ingelheim, Lilly, Merck Sharp Dhôme and Novo-Nordisk.
REFERENCES
- 1. Fernandez-Fernandez B, Fernandez-Prado R, Górriz JL. et al. Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation and study of diabetic nephropathy with atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan? Clin Kidney J 2019; 12: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vergara A, Jacobs-Cachá C, Soler MJ.. Sodium-glucose cotransporter inhibitors: beyond glycaemic control. Clin Kidney J 2019; 12: 322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 5. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 9. Gæde P, Vedel P, Larsen N. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393 [DOI] [PubMed] [Google Scholar]
- 10. Dekkers CCJ, Wheeler DC, Sjöström CD. et al. Effects of the sodium–glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and stages 3b–4 chronic kidney disease. Nephrol Dial Transplant 2018; 33: 1280–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh M, Kumar A.. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf 2018; 13: 84–91 [DOI] [PubMed] [Google Scholar]
- 12. Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl 2011; 79: S14–S19 [DOI] [PubMed] [Google Scholar]
- 13. Yang Y, Chen S, Pan H. et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes. Medicine (Baltimore) 2017; 96:e6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFronzo RA, Ferrannini E, Groop L. et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015; 1: 15019. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 16. Jardine MJ, Mahaffey KW, Neal B. et al. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017; 46: 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkovic V, de Zeeuw D, Mahaffey KW. et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol 2018; 6: 691–704 [DOI] [PubMed] [Google Scholar]
- 18. Szablewski L. Distribution of glucose transporters in renal diseases. J Biomed Sci 2017; 24: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navale AM, Paranjape AN.. Glucose transporters: physiological and pathological roles. Biophys Rev 2016; 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Armando I, Upadhyay K. et al. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens 2009; 18: 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Umino H, Hasegawa K, Minakuchi H. et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep 2018; 8: 6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pessoa TD, Campos LCG, Carraro-Lacroix L. et al. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 2014; 25: 2028–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung S, Kim S, Son M. et al. Empagliflozin contributes to polyuria via regulation of sodium transporters and water channels in diabetic rat kidneys. Front Physiol 2019; 10: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Q, Steiger S, Anders HJ.. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol Rep 2017; 5: e13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Thai K, Kepecs DM. et al. Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS One 2016; 11: e0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abbas NAT, El. Salem A, Awad MM.. Empagliflozin, SGLT2inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol 2018; 391:1347–1360. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Nakano D, Guan Y. et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int 2018; 94: 524–535 [DOI] [PubMed] [Google Scholar]
- 28. Cassis P, Locatelli M, Cerullo D. et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018; 3: e98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panchapakesan U, Pegg K, Gross S. et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heerspink HJL, Perco P, Mulder S. et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019; 62: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cefalu WT, Leiter LA, Yoon KH. et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950 [DOI] [PubMed] [Google Scholar]
- 33. Pirklbauer M, Schupart R, Fuchs L. et al. Unraveling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am J Physiol Renal Physiol 2019; 316: F449–F462 [DOI] [PubMed] [Google Scholar]
- 34. Filippatos TD, Liontos A, Papakitsou I. et al. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med 2019; 131: 82–88 [DOI] [PubMed] [Google Scholar]
- 35. Wright EM, Hirayama BA, Loo DF.. Active sugar transport in health and disease. J Intern Med 2007; 261: 32–43 [DOI] [PubMed] [Google Scholar]
- 36. Zhou L, Cryan EV, D'Andrea MR. et al. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem 2003; 90: 339–346 [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Williams S, Ho S. et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diab Ther 2010; 1: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tazawa S, Yamato T, Fujikura H. et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1, 5-anhydro-D-glucitol, and fructose. Life Sci 2005; 76: 1039–1050 [DOI] [PubMed] [Google Scholar]
- 39. You G, Lee WS, Barros EJG. et al. Molecular characteristics of Na+-coupled glucose transporters in adult and embryonic rat kidney. J Biol Chem 1995; 270: 29365–29371 [DOI] [PubMed] [Google Scholar]
- 40. Ng KM, Lau YM, Dhandhania V. et al. Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci Rep 2018; 8: 14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byrne NJ, Parajuli N, Levasseur JL. et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci 2017; 2: 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uthman L, Nederlof R, Eerbeek O. et al. Delayed ischemic contracture onset by Empagliflozin associates with NHE-1 inhibition and is dependent on insulin in isolated mouse hearts. Cardiovasc Res 2019; 115: 1533. [DOI] [PubMed] [Google Scholar]
- 43. Uthman L, Baartscheer A, Bleijlevens B. et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018; 61: 722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bookstein C, DePaoli AM, Xie Y. et al. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 1994; 93: 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mustroph J, Lücht CM, Wagemann O. et al. Empagliflozin enhances human and murine cardiomyocyte glucose uptake by increased expression of GLUT1. Diabetologia 2019; 62: 726–729 [DOI] [PubMed] [Google Scholar]
- 46. Saponaro C, Pattou F, Bonner C.. SGLT2 inhibition and glucagon secretion in humans. Diabet Metab 2018; 44: 383–385 [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency (EMA). Invokana product information: https://www.ema.europa.eu/en/documents/product-information/invokana-epar-product-information_en.pdf; Jardiance product information: https://www.ema.europa.eu/en/documents/product-information/jardiance-epar-product-information_en.pdf; Forxiga product information: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf (27 October 2019, date last accessed)
- 48.U.S. Food & Drug Administration (FDA). Invokana medication guide: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204042s032lbl.pdf#page=49; Jardiance medication guide: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204629s019lbl.pdf#page=34; Farxiga medication guide: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202293s018lbl.pdf#page=38 (27 October 2019, date last accessed).
- 49. Piepoli MF, Hoes AW, Agewall S. et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davies MJ, D’Alessio DA, Fradkin J. et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61: 2461–2498 [DOI] [PubMed] [Google Scholar]
- 51. Sarafidis P, Ferro CJ, Morales E. et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019; 34: 208–230 [DOI] [PubMed] [Google Scholar]
- 52. Mosenzon O, Wiviott SD, Cahn A. et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabet Endocrinol 2019; 7: 606–617. [DOI] [PubMed] [Google Scholar]
- 53. Zoccali C, Blankestijn PJ, Bruchfeld A. et al. Children of a lesser god: exclusion of chronic kidney disease patients from clinical trials. Nephrol Dial Transplant 2019; 34: 1112. [DOI] [PubMed] [Google Scholar]
- 54. Macha S, Mattheus M, Halabi A. et al. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab 2014; 16: 215–222 [DOI] [PubMed] [Google Scholar]
- 55. Nadkarni GN, Ferrandino R, Chang A. et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care 2017; 40: 1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blau JE, Tella SH, Taylor SI. et al. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev 2017; 33: e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li D, Wang T, Shen S. et al. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 2017; 19: 348–355 [DOI] [PubMed] [Google Scholar]
- 58. Tang H, Dai Q, Shi W. et al. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017; 60: 1862–1872 [DOI] [PubMed] [Google Scholar]
- 59. Ruanpeng D, Ungprasert P, Sangtian J. et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev 2017; 33: e2903. [DOI] [PubMed] [Google Scholar]
- 60. Singh M, Sharma R, Kumar A.. Safety of SGLT2 inhibitors in patients with diabetes mellitus. Curr Drug Saf 2019; 14: 87–93 [DOI] [PubMed] [Google Scholar]
- 61. Donnan JR, Grandy CA, Chibrikov E. et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open 2019; 9: e022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matthews DR, Li Q, Perkovic V. et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS program. Diabetologia 2019; 62: 926–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bersoff-Matcha SJ, Chamberlain C, Cao C. et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med 2019; 170: 764. [DOI] [PubMed] [Google Scholar]
- 64. Davies MJ, D’Alessio DA, Fradkin J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bilo H, Coentrao L, Couchoud C. et al. Clinical practice guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR < 45 mL/min). Nephrol Dial Transplant 2015; 30: ii1–ii142 [DOI] [PubMed] [Google Scholar]
- 66. Castro Conde A, Marzal D, Arrarte V. et al. Abordaje integral del paciente con diabetes mellitus tipo 2 y enfermedad cardiovascular o de muy alto riesgo cardiovascular. Cardiclinics 2019; 54: 183–192 [Google Scholar]
- 67. Yale JF, Bakris G, Cariou B. et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014; 16: 1016–1027 [DOI] [PubMed] [Google Scholar]
- 68. Álvarez F, Alemán JJ. Inhibidores del cotransportador de sodio-glucosa tipo 2. Diabetes práctica 2018; 9: 1–24: http://www.diabetespractica.com/files/1518601321.03_alvarez-sp_9-1.pdf (27 October 2019, date last accessed) [Google Scholar]
- 69. Jabbour SA, Frías JP, Hardy E. et al. Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care 2018; 41: 2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lim S. Effects of sodium‐glucose cotransporter inhibitors on cardiorenal and metabolic systems: latest perspectives from the outcome trials. Diabetes Obes Metab 2019; 21: 5–8 [DOI] [PubMed] [Google Scholar]
- 71. Bonora BM, Avogaro A, Fadini GP.. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diabetes Obes Metab 2018; 20: 25–33 [DOI] [PubMed] [Google Scholar]
- 72. Danne T, Garg S, Peters AL. et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019; 42: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.