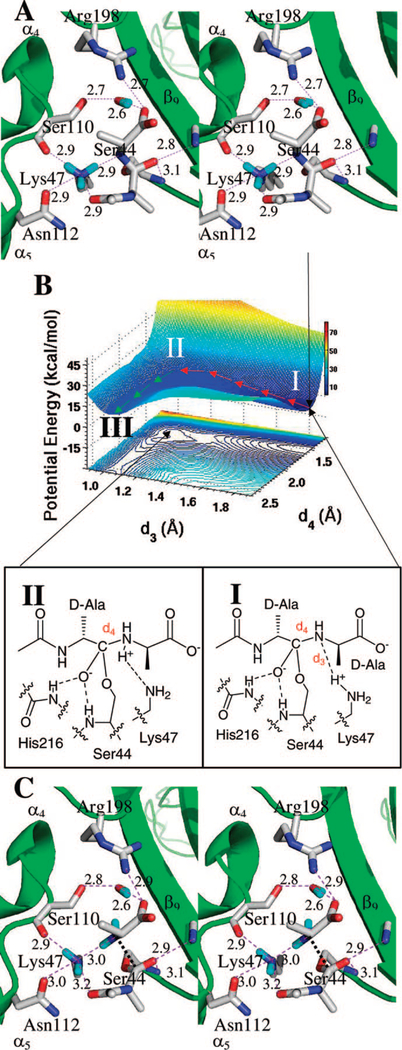
Figure 3.
(A) Stereo representation of the tetrahedral intermediate in the reaction to acyl-enzyme through direct Lys47 protonation. Hydrogen bonds are shown as dashed lines (distances in Å between heteroatoms). The tetrahedral species is nestled between β9 sheet and the loop connecting α4 and α5 helices, with the protein depicted as the ribbon representation in green. Important active site residues and the tetrahedral intermediate are represented in capped-stick. Carbon atoms are colored in gray, nitrogen in blue, oxygen in red, and hydrogen in cyan. Distances (Å) are rounded to the nearest tenth. (B) QM/MM potential-energy surface and the contour over reaction coordinates represented as a shadow. The reaction paths are shown by red and green arrows from the tetrahedral intermediate (I) to an intermediate complex (II), and to the acyl-enzyme (III). The energy surface constructed using the complex coordinates (d3–dNH) vs d4 is qualitatively identical. The distance dNH is the distance between the lysine NH atoms. (C) The structure of the acyl-enzyme III is shown. The general description given for panel A applies here as well.