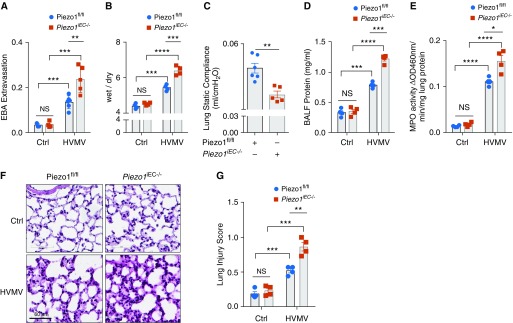
Figure 1.
Piezo1 deletion in ECs of mice augments high-volume mechanical ventilation (HVMV)-induced lung capillary leakage. (A and B) Endothelium-specific genetic deletion of Piezo1 (Piezo1iEC−/−) augments HVMV-induced pulmonary capillary leakage and edema formation. Piezo1fl/fl and Piezo1iEC−/− mice were mechanically ventilated with tidal volume of 40 ml/kg for 2 hours. Pulmonary transvascular albumin permeability (A) and wet/dry ratio (B) were measured. Plots show mean ± SEM. The data points depict individual mice; n = 3–5. **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA. (C) Piezo1iEC−/− decreases lung static compliance in mice undergoing mechanical ventilation. Values are shown as mean ± SEM; n = 5–6. **P < 0.01 by two-tailed t test. (D) Piezo1iEC−/− mice show an increase in BAL fluid (BALF) protein concentration in mice undergoing HVMV as in A and B. Mean ± SEM; n = 3–4. ***P < 0.001 and ****P < 0.0001 by ANOVA. (E) Piezo1iEC−/− mice show an increase in HVMV-induced neutrophil sequestration in lungs (assessed by measurement of myeloperoxidase [MPO] activity). Mean ± SEM; n = 4. *P < 0.05 and ****P < 0.0001 by ANOVA. (F and G) Hematoxylin and eosin staining of lung sections (F) and Lung Injury Score (G) showing increased inflammation and lung injury in Piezo1EC−/− mice postventilation as in A and B. Scale bars: 60 μm. **P < 0.01 and ***P < 0.001 by ANOVA. Ctrl = control; EBA = Evans blue-labeled albumin; ECs = endothelial cells; NS = nonsignificant; OD = optical density.